Researchers in Germany have confirmed that hydrogen sulphide - the compound that gives rotten eggs and farts their distinctive odour - can conduct electricity without resistance at a record-breaking –70 °C. Smashing the previous record of around –110 °C, the research draws us ever-closer to the dream of a room-temperature superconductor that could one day change everything about how we generate and transmit energy.
The ability to achieve superconductivity without cooling would not only revolutionise the technologies that already rely on it, such as maglev trains, MRI machines, and particle accelerators, but suddenly all of our electronic devices would be rendered ultra-efficient. Losing zero energy to resistance without the need for industrial cooling would mean that everything from wind turbines to electrical motors and laptop chargers would require significantly less electricity to run.
The team from the Max Planck Institute for Chemistry got their first hints that hydrogen sulphide was worth investigating back in December, and now report that when they used a diamond anvil to put samples of hydrogen sulphide under extreme amounts of pressure - around 1.5 million atmospheres (150 gigapascals) - and cooled them to –70 °C, they transformed from a gas to a metal and achieved superconductivity.
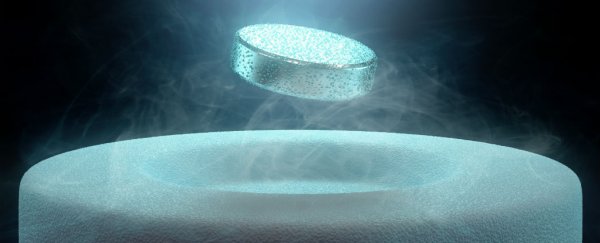
Superconductivity is defined by two main characteristics: zero electrical resistance and something known as the Meissner effect. The Meissner effect occurs when a material makes the transition from a normal to a superconducting state and excludes magnetic fields from its interior. This phenomenon is what causes magnets to levitate above a superconductor.
Not only is the relatively high temperature used to achieve this state in hydrogen sulphide exciting, Edwin Cartlidge reports at Nature Magazine that scientists are also super interested in the result "because it was achieved without using exotic materials such as the copper-containing compounds called 'cuprates', which until now have held the record for the highest superconducting temperature –140 °C at ambient pressure and –109 °C at high pressure".
While the team isn't entirely sure why their hydrogen sulphide superconductor works, they hypothesise in their Nature paper that it could have to do with the material being packed with hydrogen ions, which 'encourage' electrons to form what's known as Cooper pairs. These Cooper pairs can flow through the crystal lattice structure of the newly formed hydrogen sulphide metal without resistance, which allows an electric current to flow through more quickly.
Colin Barras explains at New Scientist:
"Electrons traveling through a metal constantly ricochet off ions, losing energy with each bounce. However, in the process they slightly shift the position of positive ions in the metal, generating small clouds of positive charge. These positive clouds can pull electrons together, and result in the formation of Cooper pairs, which are much less likely to bump into metal ions and lose energy. Because of this, the electron pairs conduct a charge far more efficiently than single electrons."
Usually, Cooper pairs are easily broken apart by heat, which is why the superconductors of today need to be cooled to very low temperatures. As Barras reports, the new Max Planck superconductor can work at higher temperatures because its positive ions include light hydrogen, and this is more easily shifted into pairs by the electrons. "This means the positive clouds are denser, and the electrons form stronger Cooper pairs that are less easily broken by heat," he says.
Before we get too excited, we have to wait for the results to be replicated by an independent team. Cartlidge reports at Nature Magazine that a team in Japan has achieved a loss of resistance in pressurised hydrogen sulphide, but has so far seen no signs of the Meissner effect. And three teams in China and one in the US, all of which have been working with hydrogen sulphide, have yet to confirm either.
One of the Max Planck team, Mikhail Eremets, told New Scientist he hopes someone will not only confirm their new record, but beat it. He notes that there are materials out there that can produce even tougher Cooper pairs, and that's how we can get closer to the elusive room-temperature superconductor. "Theoretically, they are not forbidden," he says.