Scientists have developed a thin flexible battery suited to implantable devices that does away with dangerous chemicals and replaces them with biologically compatible liquids.
The bendy batteries use sodium-based liquids – one a saline solution and another using cell cultures – and this innovation could change how we power wearable devices and even medical implants.
"Current batteries like the lithium-ion ones used in medical implants generally come in rigid shapes," says one of the researchers, Yonggang Wang from Fudan University in China.
"Additionally, most of the reported flexible batteries are based on flammable organic or corrosive electrolytes, which suffer from safety hazards and poor biocompatibility for wearable devices, let alone implantable ones."
The demand for wearable and implantable devices such as bracelets, wearable sensors and electronic pills has led to an increased research effort to create miniature batteries to power them.
We've all witnessed the mess and corrosion leaky batteries can cause in our precious electronic equipment. Miniaturised batteries using these chemicals need significant structural reinforcement and substantial sealing to ensure the hazardous chemicals don't leak out.
The best solution: get rid of the corrosive electrolyte altogether.
The research team swapped the corrosive chemicals for sodium-based liquids which are completely bio-compatible. One option used a normal saline solution and another a cell culture medium containing amino acids, sugars, and vitamins – a liquid that mimics the fluid around cells in the human body.
With the liquids posing no harm to the interior of the body, the danger of electrolyte leakage is greatly minimised.
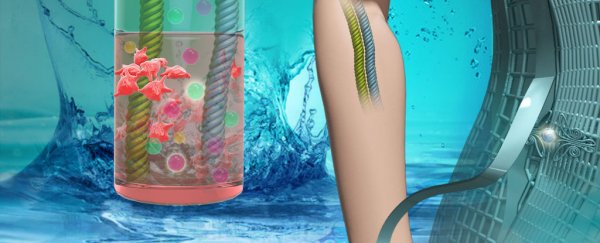
The team made two types of batteries. The first used thin films of electrode material stuck on a mesh made of steel strands, creating a flat "2D" belt. The second used a woven carbon nanotube fibre backbone with embedded nanoparticle electrode materials.
Both styles can be seen in the illustration below.
 The two flexible battery concepts. Credit: Zhaowei Guo, et. al.
The two flexible battery concepts. Credit: Zhaowei Guo, et. al.
Both battery types outperformed most of the current lithium-ion batteries used in wearable electronics in terms of the amount of charge they can hold and power output – the two crucial factors that determine a battery's success.
But then the carbon nanotube electrode also did something unexpected.
During testing, the scientists noticed an interesting side reaction. The carbon nanotube backbone accelerated the conversion of dissolved oxygen into hydroxide ions. While this was a process that destroys the effectiveness of the battery, it is perfect for cancer starvation therapy.
Although this observation is exciting, it will take more interdisciplinary research to confirm that this is a viable treatment for cancer, so it remains little more than a theoretical application at the moment.
"We can implant these fibre-shaped electrodes into the human body to consume essential oxygen, especially for areas that are difficult for injectable drugs to reach," says Wang.
"Deoxygenation might even wipe out cancerous cells or pathogenic bacteria since they are very sensitive to changes in living environment pH."
There's no doubt the batteries have some impressive functionality, but they'll require further testing before we can be sure they will stand up to the pressure of life in the kinds of electronics they would be useful for.
The results were reported in Chem.