It's taken decades to nail down, but researchers in Canada have finally identified a new chemical bond, which they're calling a 'vibrational bond'.
This vibrational bond seems to break the law of chemistry that states if you increase the temperature, the rate of reaction will speed up. Back in 1989, a team from the University of British Columbia investigated the reactions of various elements to muonium (Mu) - a strange, hydrogen isotope made up of an antimuon and an electron. They tried chlorine and fluorine with muonium, and as they increased the heat, the reaction time sped up, but when they tried bromine (br), a brownish-red toxic and corrosive liquid, the reaction time sped up as the temperature decreased. The researchers, Amy Nordrum writes for Scientific American, "were flummoxed".
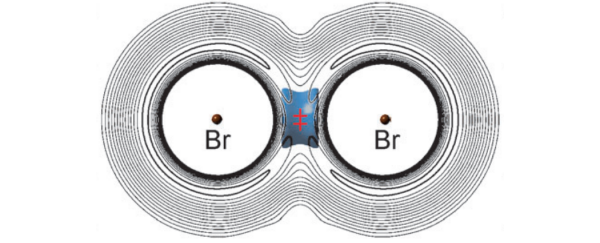
Perhaps, thought one of the team, chemist Donald Flemming, when the bromine and muonium made contact, they formed a transitional structure made up of a lightweight atom flanked by two heavier atoms. And the structure was joined not by van der Waal's forces - as would usually be expected - but by some kind of temporary 'vibrational' bond that had been proposed several years earlier.
"In this scenario, the lightweight muonium atom would move rapidly between two heavy bromine atoms, 'like a Ping Pong ball bouncing between two bowling balls,' Fleming says. The oscillating atom would briefly hold the two bromine atoms together and reduce the overall energy, and therefore speed, of the reaction."
But back then, the team didn't have the technology needed to actually see this reaction take place, because it lasts for just a few milliseconds. But now they do, and the team took their investigation to the nuclear accelerator at Rutherford Appleton Laboratory in England.
With the help of theoretical chemists from the Free University of Berlin and Saitama University in Japan, Flemming's team watched as the light muonium and heavy bromine formed a temporary bond. "The lightest isotopomer, BrMuBr, with Mu the muonium atom, alone exhibits vibrational bonding in accord with its possible observation in a recent experiment on the Mu + Br2 reaction," the team reports in the journal Angewandte Chemie International Edition. "Accordingly, BrMuBr is stabilised at the saddle point of the potential energy surface due to a net decrease in vibrational zero point energy that overcompensates the increase in potential energy."
In other words, the vibration in the bond decreased the total energy of the BrMuBr structure, which means that even when the temperature was increased, there was not enough energy to see an increase in the reaction time.
While the team only witnessed the vibrational bond occurring in a bromine and muonium reaction, they suspect it can also be found in interactions between lightweight and heavy atoms, where van der Waal's forces are assumed to be at play.
"The work confirms that vibrational bonds - fleeting though they may be - should be added to the list of known chemical bonds," says Nordrum at Scientific American.
Sorry, future high school chemistry students, here's another thing you'll probably have to rote learn.
Source: Scientific American