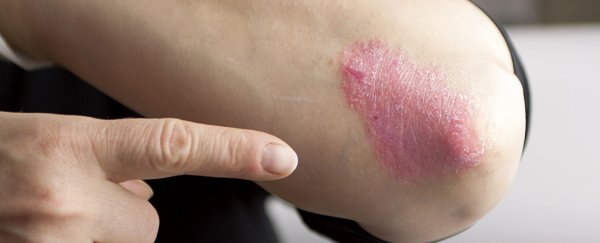
Psoriasis shows itself as red and blotchy skin (often around the elbows and knees) and is caused by an abnormal build-up of skin cells. Its severity varies from person to person, and it's a chronic condition for which there is no cure. But that could now change, thanks to a new drug developed by scientists in the US.
After three long-term clinical trials, 70-80 percent of psoriasis patients saw it completely or almost completely cured - an impressive result for such a stubborn disease.
The drug is called ixekizumab, and it offers a new hope for a psoriasis-free future for the 3 percent of the world's population living with the condition.
It's not just the skin irritation and psychological damage caused by this very visible disease that people have to deal with - psoriasis is also linked to an increased risk of depression, heart disease, and diabetes.
"This group of studies not only shows very high and consistent levels of safety and efficacy, but also that the great majority of the responses persist at least 60 weeks," said one of the team, dermatologist Kenneth Gordon, from the Northwestern University Feinberg School of Medicine.
What makes the research so promising is the number of people involved: 3,736 adult patients across more than 100 study sites in 21 different countries. All those involved had moderate to severe psoriasis, which means more than 10 percent of their bodies were covered.
By the 60th week, the psoriasis was classified as 'clear' or 'minimal' for 76.4 to 81.8 percent of the participants, depending on the study group. That compares very favourably to the 3.2 percent figure recorded for the control group on placebos.
"Ten years ago, we thought complete clearance of this disease was impossible," says Gordon. "It wasn't something we would even try to do. Now with this drug, we're obtaining response levels higher than ever seen before."
Based on the findings of the study, Gordon says he would expect 80 percent of patients to have an "extremely high response rate" to ixekizumab, while about 40 percent will find their disease completely cleared up.
Ixekizumab works by neutralising a pathway in the immune system that's known to promote psoriasis, and the drug has now been approved by the Food and Drug Administration (FDA) in the US.
But there are some side effects to consider, including slightly higher rates of neutropenia (low white blood cell count), yeast infection, and inflammatory bowel disease.
Monitoring will continue beyond 60 weeks for patients on the treatment so the researchers can get a better understanding of these side effects.
The results of the trial have been published in The New England Journal of Medicine.