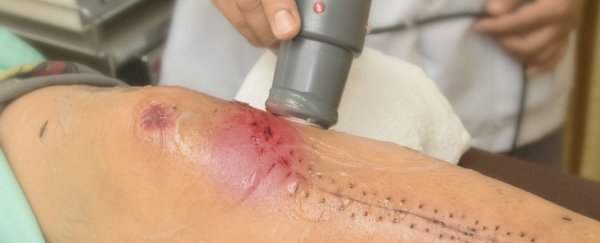
A new medical device that can accelerate the body's capacity to heal wounds by up to 30 percent has been developed by researchers in the UK, and it works by tricking the skin cells into behaving much younger than they actually are. That gash you got from playing football? Imagine it healing as fast as if you were a kid in high school.
Ultrasound is the secret ingredient in the device: it produces nano-vibrations in the skin cells surrounding the wound, opening up channels within the skin's cellular membrane and releasing the flow of calcium. This flow then pulls the cells towards the damaged part of the body and causes the wound to heal faster. While it's not quite at the level of the miraculous healing scanners seen in science-fiction movies, it's getting us one step closer.
According to the team behind the technology, it works especially well for diabetics and the elderly - groups of people whose bodies usually take longer to heal naturally. According to estimates, the UK National Health Service could save some £3.1 billion a year as patients recover more quickly and free up the time of doctors and nurses, though this technology is still several years away from being put into practice.
"Using ultrasound wakes up the cells and stimulates a normal healing process," says lead author Mark Bass from Sheffield University. "Because it is just speeding up the normal processes, the treatment doesn't carry the risk of side effects that are often associated with drug treatments." He adds that the treatment could be particularly effective on chronic, long-standing wounds and skin ulcers.
If additional rounds of testing go well, the ultrasound device developed by Bass and his team could be used on patients in the next three or four years. "Now that we have proven the effectiveness of ultrasound we need to explore the signal further," he says. "We have found that the ultrasound signal we currently use is effective, but it is possible that by refining the treatment we could improve the effects even further."
Ultrasound is not a new technology, though it's being used in a new way here. That means researchers already have plenty of older reports and studies they can refer back to as they try and develop the device further, which should also come in handy when submitting the technology to regulatory boards such as the US Food and Drug Administration (FDA) and the UK Medicines and Healthcare Products Regulatory Agency (MHRA) for public use.