Say hello to ionocaloric cooling. It's a new way to lower temperatures with the potential to replace existing methods of chilling things with a process that is safer and better for the planet.
Typical refrigeration systems transport heat away from a space via a fluid that absorbs heat as it evaporates into a gas, which is then transported through a closed tube and condensed back into a liquid. As effective as this process is, some of the choice materials we use as refrigerants are particularly unfriendly to the environment.
There is, however, more than one way a substance can be forced to absorb and shed heat energy.
A method developed by researchers from the Lawrence Berkeley National Laboratory and the University of California, Berkeley, in the US takes advantage of the way that energy is stored or released when a material changes phase, as when solid ice turns to liquid water, for example.
Raise the temperature on a block of ice, it'll melt. What we might not see so easily is that melting absorbs heat from its surroundings, effectively cooling it.
One way to force ice to melt without needing to turn up the heat is to add a few charged particles, or ions. Putting salt on roads to prevent ice from forming is a common example of this in action. The ionocaloric cycle also uses salt to change a fluid's phase and cool its surroundings.
"The landscape of refrigerants is an unsolved problem," said mechanical engineer Drew Lilley from the Lawrence Berkeley National Laboratory in California in January 2023 statement. "No one has successfully developed an alternative solution that makes stuff cold, works efficiently, is safe, and doesn't hurt the environment."
"We think the ionocaloric cycle has the potential to meet all those goals if realized appropriately."
The researchers modeled the theory of the ionocaloric cycle to show how it could potentially compete with, or even improve upon, the efficiency of refrigerants in use today. A current running through the system would move the ions in it, shifting the material's melting point to change temperature.
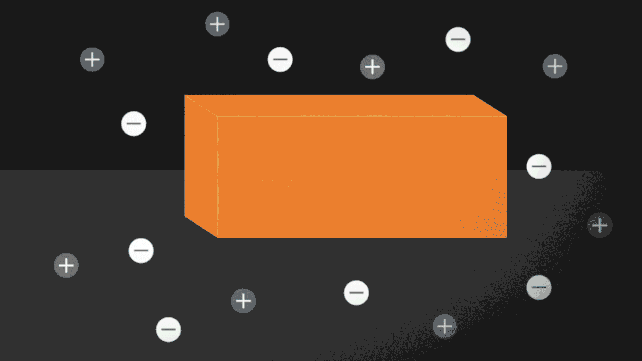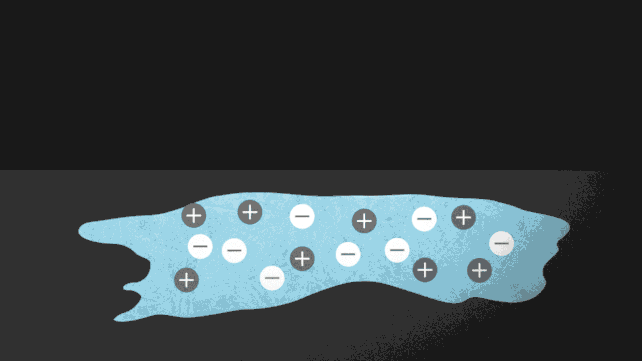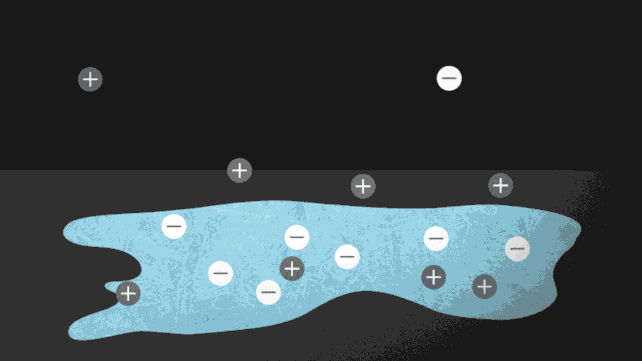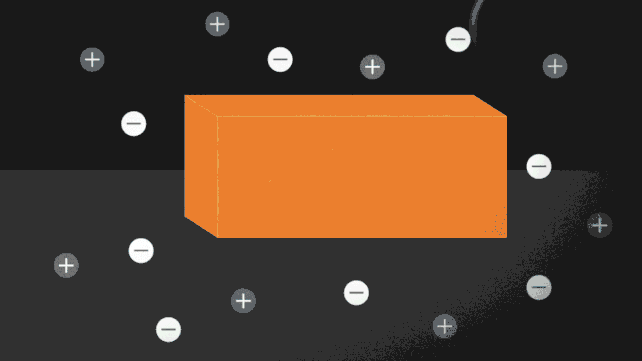
The team also ran experiments using a salt made with iodine and sodium to melt ethylene carbonate. This common organic solvent is also used in lithium-ion batteries and is produced using carbon dioxide as an input. That could make the system not just GWP [global warming potential] zero but GWP negative.
A temperature shift of 25 degrees Celsius (45 degrees Fahrenheit) was measured through the application of less than a single volt of charge in the experiment, a result that exceeds what other caloric technologies have managed to achieve so far.
"There are three things we're trying to balance: the GWP of the refrigerant, energy efficiency, and the cost of the equipment itself," said mechanical engineer Ravi Prasher from the Lawrence Berkeley National Laboratory.
"From the first try, our data looks very promising on all three of these aspects."
The vapor compression systems currently used in refrigeration processes rely on gases that have high GWP, such as various hydrofluorocarbons (HFCs). Countries that signed up to the Kigali Amendment have committed to reducing the production and consumption of HFCs by at least 80 percent over the next 25 years – and ionocaloric cooling could play a major part in that.
Now, the researchers need to get the technology out of the lab and into practical systems that can be used commercially and that scale up without any issues. Eventually, these systems could be used for heating as well as cooling.
"We have this brand-new thermodynamic cycle and framework that brings together elements from different fields, and we've shown that it can work," said Prasher.
"Now, it's time for experimentation to test different combinations of materials and techniques to meet the engineering challenges."
The research was published in Science.
A version of this article was first published in January 2023.
