Scientists have developed a new tool for determining the age of eye cells without sampling regenerative tissue, which could make treatments for eye disease more personalized and better targeted.
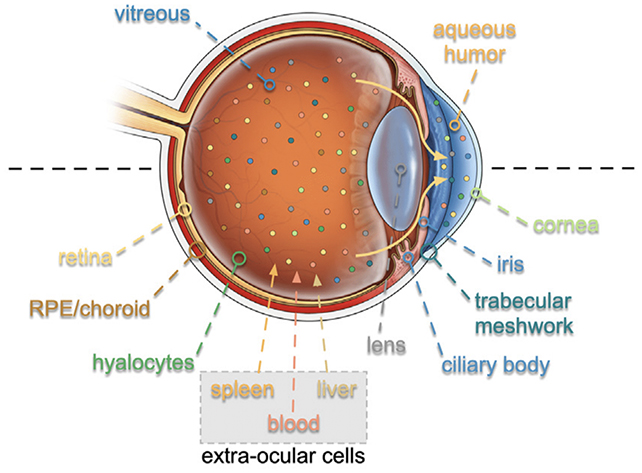
The team, led by researchers from Stanford University, adapted a technique used for analyzing eye fluid. Almost 6,000 proteins detected in the fluid could be traced back to the eye's cells, 26 of which were collectively linked to eye aging.
This was done through an artificial intelligence system trained on the eye fluid of 46 healthy patients, where ages could be cross-referenced. In further tests, the AI was able to predict someone's age from their eye fluid (give or take a few years).
"The first step in developing any kind of successful therapy is understanding the molecules," says ophthalmologist Vinit Mahajan, from Stanford University.
Having established their eye-aging clock, the researchers looked at how specific eye diseases could accelerate cell aging. A further analysis was run on eye fluid from 62 patients with different types of eye disease.
Certain proteins showed higher levels of aging: patients with early-stage diabetic retinopathy (damage to retinal cells due to a decrease in blood supply) were predicted to be able 12-years older based on the tests compared with healthy patients, for example. For patients with uveitis (inflammation inside the eye), that 'jump' was close to 30 years.
Using an approach called TEMPO – for 'tracing expression of multiple protein origins' – the team traced the affected proteins back to the RNA (ribonucleic acid) that created them, highlighting the problematic cells responsible for these diseases.
The cells responsible were found to be different for each disease, and not always the most obvious cells that are usually targeted by treatments, potentially giving scientists new targets when it comes to treating these kinds of problems.
"At the molecular level, patients present different manifestations even with the same disease," says Mahajan. "With a molecular fingerprint like we've developed, we could pick drugs that work for each patient."
These new techniques get over a perennial problem for studying diseases in the eye, and other areas like the brain: taking tissue samples from these particular parts of the body can cause a significant amount of damage, as they have a harder time regenerating the lost cells.
What's more, the researchers think the innovations they've demonstrated here could work with other types of bodily fluids, giving experts invaluable biomarkers to treat and perhaps even prevent the development of diseases.
"It's as if we're holding these living cells in our hands and examining them with a magnifying glass," says Mahajan.
"We're dialing in and getting to know our patients intimately at a molecular level, which will enable precision health and more informed clinical trials."
The research has been published in Cell.