A bitter taste is unmistakable, but what chemical processes cause it? Thanks to new research, we now know how our tongue's taste receptors (taste buds) are activated at the molecular level, a breakthrough in the science of taste.
Led by a team from the University of North Carolina (UNC) School of Medicine, the study focuses on a bitter taste receptor called TAS2R14, and its role in helping identify one of the five different tastes we can sense: sweet, sour, salty, bitter, and umami (or savory).
It builds on what we know about the sense of taste, and could potentially lead to improved treatments for health conditions that the TAS2R14 receptor has been implicated in – including obesity, diabetes, asthma, and chronic obstructive pulmonary disease.
"Scientists know very little about the structural make up of sweet, bitter, and umami taste receptors," says pharmacologist Yoojoong Kim, from the UNC School of Medicine.
"Using a combination of biochemical and computational methods, we now know the structure of the bitter taste receptor TAS2R14 and the mechanisms that initialize the sensation of bitter taste in our tongues."
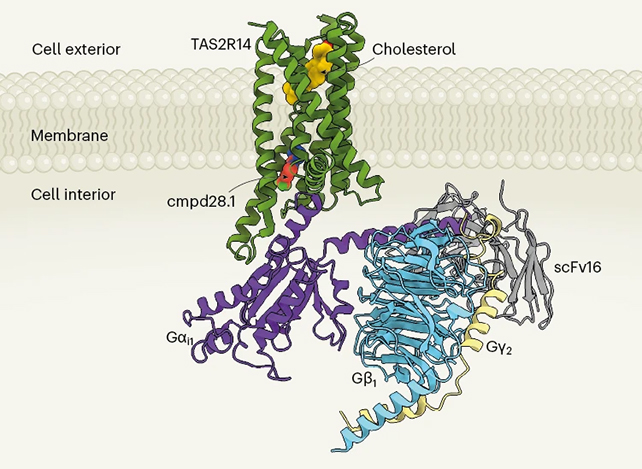
Those methods included cryogenic electron microscopy, which allows ultra-precise imaging of the 3D structural biology of molecules in their active state by rapidly preserving them at ultra-cold temperatures. Scientists can then study the function of molecules like proteins and viruses, which can lead to more targeted treatments for conditions that the molecule plays a role in.
The team revealed that when bitter substances (or tastants) hit TAS2R14, they're wedged into the allosteric site. This regulatory region allows molecules to bind to a protein and influence its functional activity.
The mechanism hasn't been discovered before. The tastant's connection with the allosteric site changes the shape of the receptor, activating its coupled G protein and setting off a chain reaction of signaling further down the line.
This is how the message gets sent to a part of the brain called the gustatory cortex, where we process and perceive the signals as the taste of bitterness.
Another new discovery is the involvement of cholesterol, which binds to the active site on TAS2R14 to help trigger the process of detecting the bitter taste. Bile acids, which have a similar structure to cholesterol, have previously been linked to TAS2R14 activation.
"Cholesterol was residing in another binding site called the orthosteric pocket in TAS2R14, while the bitter tastant binds to the allosteric site," says Kim.
"Through molecular dynamics simulations, we also found that the cholesterol puts the receptor in a semi-active state, so it can be easily activated by the bitter tastant."
As for how the new findings might be used, beyond a better understanding of the body's most intricate processes, it may seem a leap from tasting something bitter to the health issues we mentioned earlier.
However, these receptors are also found in our airways and play a role in appetite regulation, while cholesterol and bile acids help control metabolism – so treatments for any related problems could benefit from what scientists have learned here.
"In the future, this structure will be key to discovering and designing drug candidates that can directly regulate G proteins through the allosteric sites," says Kim.
The research has been published in Nature.
