As strange and unique as the laws of the quantum realm appear in our everyday experience, every now and then experiments catch sight of phenomena that seem both alien and yet eerily familiar.
For the first time, droplets have been seen shattering into a 'quantum rain' in a degenerate, ultracold fluid of potassium and rubidium isotopes, bridging the classical world of fluid dynamics with the ethereal landscape of atomic gases.
A team of researchers from across Spain and Italy analyzed the properties of fragmenting atomic gas, expanding on current understanding of how quantum liquids behave in ways that might help us better manipulate their activity.
"Our measurements not only advance the understanding of this exotic liquid phase, but also demonstrate the possibility of creating quantum droplet arrays for future applications in quantum technologies," says Luca Cavicchioli, first author of the study and condensed matter physicist from Italy's National Institute of Optics.
Watching a storm send showers of water droplets cascading against a window pane is poetry written in notes of physics. Every liquid sphere is bound by a skin of surface tension, merging and splitting and merging again as gravity drags them zig-zagging down the glass in fits and starts.

Those movements are largely the result of a tug-of-war between molecular forces in what's known as the Plateau–Rayleigh instability.
A subtle imbalance in the charges spread across the water's hydrogen and oxygen atoms creates a dipole effect, pushing and pulling against charges in the water and glass to either split larger droplets into smaller ones or aggregate smaller ones into fat, wet beads that keep the surface area at an absolute minimum.
Where the oxygen and hydrogen atoms in water molecules are distinct systems of electrons and nuclear particles, atoms in an ultracold gas lose any sense of identity. Quantum probabilities dominate, smearing the swarming bosons into a single cloud where the concept of a point-like particle no longer makes sense.
Nonetheless, that doesn't mean there aren't competing interests within the atomic gas. In contrast to the averaging out of energy across the cloud, there are fluctuations in solutions to the cloud's potential layout, creating a repulsive nudge in what's known as a Lee-Huang-Yang correction.
This tension can also cause atomic gases to briefly flicker into smaller droplets, which vary in size and shape depending on the bosons and particle states making up the gas.
Quantum droplets have been seen and studied before, though their brief existence has made them hard to study.
The researchers behind this new experiment started with observations of quantum droplets persisting for tens of milliseconds in ultracold clouds of potassium-41 and rubidium-87, giving them a potential starting point for tinkering.
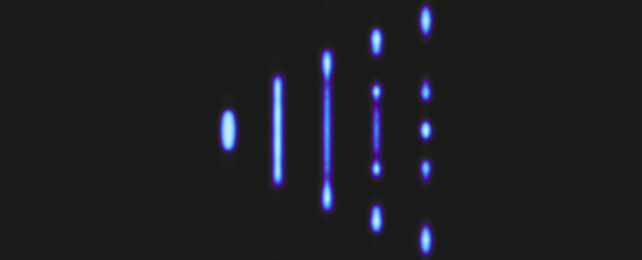
Releasing the quantum liquid into a channel called a waveguide that constricted the wave-like nature of the mixture, they found multiple droplets would form – a veritable 'rain' of activity.
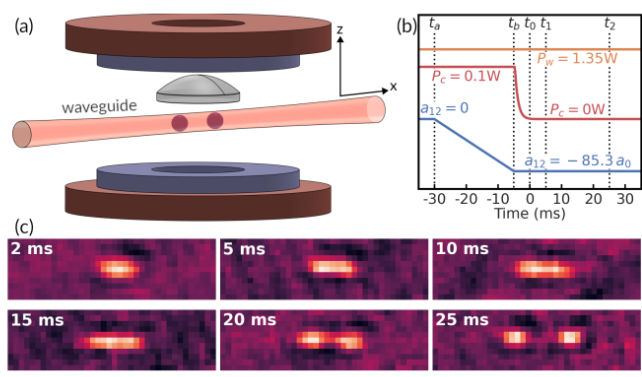
The forms the fragments took depended on their confinement into a ground state of energy, with their lengths determined by variations in atom numbers.
The dynamics of the experiment's results were predicted by theory, giving the researchers empirical grounding for new tools that could help them better understand how quantum effects mirror phenomena in our everyday lives.
"By combining experiments with numerical simulations, we were able to describe the breakup dynamics of a quantum droplet in terms of capillary instability," says University of Florence physicist Chiara Fort.
"The Plateau-Rayleigh instability is a common phenomenon in classical liquids, also observed in superfluid helium, but not yet in atomic gases."
This research was published in Physical Review Letters.