Scientists in the US have used gene editing to repair a genetic mutation in cells that causes retinitis pigmentosa, one of the leading causes of blindness in young people around the world.
Researchers employed the CRISPR technique to repair the affected cells, with the procedure representing the first time that scientists have replaced a defective gene associated with a sensory disease in stem cells that were derived from a patient's own tissue.
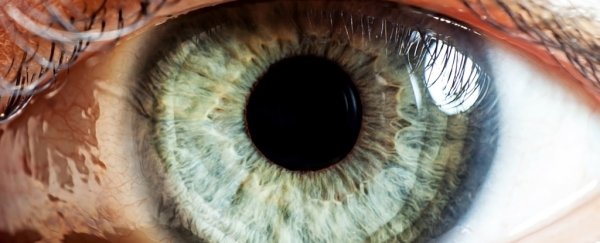
The study, published in Scientific Reports, details how the researchers took a sample of skin from a patient with retinitis pigmentosa. This inherited condition causes the retina to degrade and can lead to complete blindness within a decade.
From the skin sample, the researchers created stem cells in the lab to use as the basis for gene editing. The CRISPR technique enables scientists to cut out and replace individual components of an organism's DNA, effectively rewriting genetic code.
In this instance, the researchers took the patient-derived stem cells – which still harboured the mutation that causes retinitis pigmentosa – and used CRISPR to repair the defective gene.
As CRISPR is not approved for use in humans, that's as far as the research goes in this instance, but the advancements made here show what gene editing might be able to do for patients in the future.
According to the researchers, if the repaired stem cells were transformed into healthy retinal cells, they could be transplanted back into the patient – and without the genetic mutation, the healthy cells might then be able to restore vision loss.
"Our vision is to develop a personalised approach to treating eye disease," said ophthalmologist Stephen Tsang from Columbia University Medical Centre (CUMC). "We still have some way to go, but we believe that the first therapeutic use of CRISPR will be to treat an eye disease. Here we have demonstrated that the initial steps are feasible."
Unlike traditional organ transplants, the researchers believe a patient's body would readily accept gene-edited cells without risk of rejection by the immune system, since the repaired cells are derived from the patient's own tissue. This would also mean the powerful drugs used to suppress organ rejection would also likely not be necessary.
The researchers say optical science is the most likely first candidate for clinical use of the CRISPR technique. Compared to other parts of the body, ease of access for surgery and post-procedure monitoring makes the eye a good test case.
"Retinal diseases are a perfect model for stem cell therapy, because we have the advanced surgical techniques to implant cells exactly where they are needed," said one of the team, Vinit Mahajan, from the University of Iowa.
Due to safety and ethical concerns over the use of gene editing in humans, it's not yet clear when or if we'll see CRISPR get the green light to heal blindness in the coming years. But the scientists involved with this research are confident that their proof-of-concept shows significant potential should we go down that road.
"There is still work to do," said Tsang. "Before we go into patients, we want to make sure we are only changing that particular single mutation and we are not making other alterations to the genome."