In a world first, Australian scientists have figured out how to reprogram adult bone or fat cells to form stem cells that could potentially regenerate any damaged tissue in the body.
The researchers were inspired by the way salamanders are able to replace lost limbs, and developed a technique that gives adult cells the ability to lose their adult characteristics, multiply and regenerate multiple cell types - what is known as multipotency. That means the new stem cells can hypothetically repair any injury in the body, from severed spinal cords to joint and muscle degeneration. And it's a pretty big deal, because there are currently no adult stem cells that naturally regenerate multiple tissue types.
"This technique is a significant advance on many of the current unproven stem cell therapies, which have shown little or no objective evidence they contribute directly to new tissue formation," said lead researcher John Pimanda from the University of New South Wales, Faculty of Medicine (UNSW Medicine). "We are currently assessing whether adult human fat cells reprogrammed into [induced multipotent stem cells (iMS cells)] can safely repair damaged tissue in mice, with human trials expected to begin in late 2017."
Right now, although it's an exciting and much-hyped field of study, stem cell therapy still has a number of limitations, primarily because the most useful cells are embryonic stem cells, which are taken from developing embryos and have the potential to become any cell type in the body. But they also have the tendency to form tumours and cannot be transplanted directly to regenerate adult cells.
Instead, researchers are able to use tissue-specific adult cells, which can only turn into the cell types in their region of the body – for example, lung stem cells can only differentiate into lung tissue, so they're not as versatile as scientists need.
Scientists have also worked out how to reprogram regular adult stem cells into induced pluripotent stem cells (iPS) – a type of stem cell that's even more flexible than multipotent stem cells, but requires the use of viruses in order for the cells to be 'reset', which isn't ideal to help treat patients. That's why the new research is so exciting.
"Embryonic stem cells cannot be used to treat damaged tissues because of their tumour forming capacity," said one of the researchers, Vashe Chandrakanthan. "The other problem when generating stem cells is the requirement to use viruses to transform cells into stem cells, which is clinically unacceptable."
"We believe we've overcome these issues with this new technique."
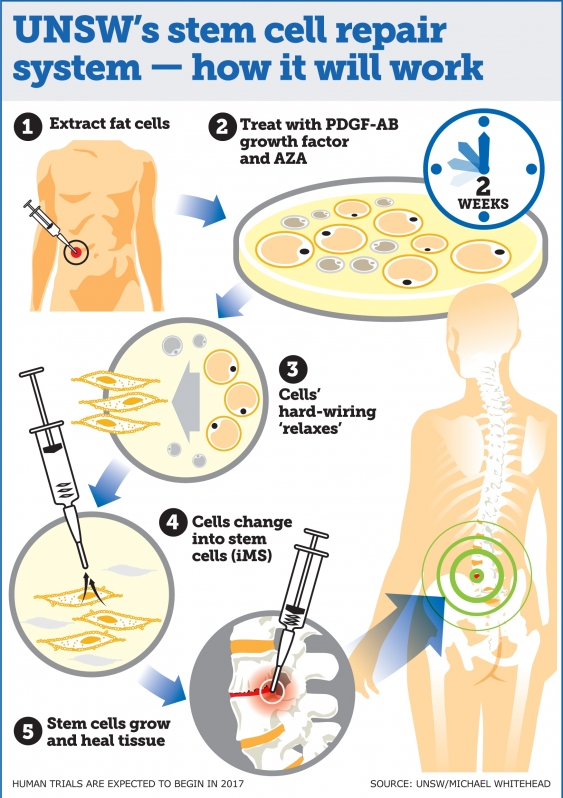
To create the new type of stem cells, the researchers collected adult human bone and fat cells and treated them with two compounds: 5-Azacytidine (AZA); and platelet-derived growth factor-AB (PDGF-AB) for two days.
This kick-started the process of dedifferentiation – which basically means it started to revert them to a multipotent stem cell state. The cells were then kept in PDGF-AB for a few weeks while they slowly changed into stem cells, eventually becoming tissue-regenerative iMS cells – which basically means they can repair any type of tissue in the body.
"This technique is ground-breaking because iMS cells regenerate multiple tissue types," said Pimanda. "We have taken bone and fat cells, switched off their memory and converted them into stem cells so they can repair different cell types once they are put back inside the body."
Right now, this process is only a proof of concept, but the researchers are already on their way to furthering the technique, and are currently investigating if human iMS cells can be transformed and repair tissue damage in mice.
The researchers also want to look into how the cells act at the sites of transplantation. If all goes well, human trials are expected for late 2017.
The first trials will focus on whether the iMS cells can heal bone, joint, and muscle tissue, helping to improve treatment for chronic back pain and injuries.
This research has been published in the Proceedings of the National Academy of Sciences.
UNSW Medicine is a sponsor of ScienceAlert. Find out more about their world-leading research.