A phase 1 trial involving a new type of RNA therapy has shown that the treatment could be used to fight psoriasis, a debilitating skin condition that affects nearly 3 percent of the world's population.
At the American Chemical Society (ACS) meeting in Philadelphia last week, researchers announced that AST-005, a type of RNA therapy, is safe to use in humans, and were optimistic about the drug's dose-dependent response in psoriasis patients.
Psoriasis is an auto-immune disease, triggered when the body creates too much of a normally healthy protein, tumour necrosis factor-α (TNF-α). The immune system attacks this protein, causing red, itchy, and scaly skin patches.
Right now, there is no cure, and very limited treatments, but RNA could be the key to controlling it.
In every one of your cells, RNA acts as a messenger between your DNA and protein. Although DNA stays in the nucleus at all times, RNA moves around the cell, directing the creation of various proteins.
One of the ways scientists have been able to limit protein creation - such as the overproduction of TNF- α - is by destroying RNA genes using a relatively new technique called RNA interference, or RNAi. RNAi enters the cell, destroys the regular RNA, and less protein is created.
While we've been able to use RNAi in animal models pretty effectively, as Robert Service explains at Science Magazine, this has been difficult to get right in humans:
"The trouble is that traditional antisense RNA drugs [synthetic RNAi] usually don't work. To date, only two antisense RNA therapies have been approved in the United States, despite decades of effort and dozens of clinical trials.
Among other problems, most introduced RNA snippets get chopped up before they reach their target by enzymes that patrol the cell for foreign material."
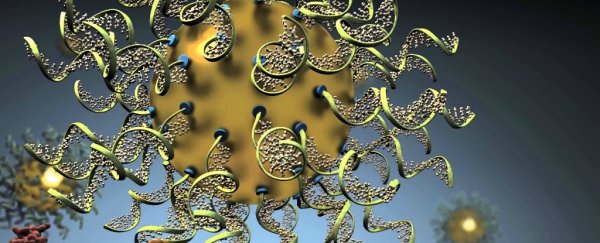
But there's renewed hope that this technique could work in humans, with the development of a new type of RNAi, called spherical nucleic acid (SNA).
Developed by researchers at US-based company, Exicure, AST-005 is a gel made up of SNAs, which has been shown in the past to lower the amount of TNF-α in animal trials. And unlike previous synthetic RNAis, the SNA's structure is not chopped up by enzymes in the cell.
Back in April, researchers from Exicure announced treatment of its first patients for a phase 1 clinical trial for the AST-005 gel, which they hoped would lower the amount of TNF-α protein produced by the corresponding gene, and therefore limit patients' symptoms.
"This clinical trial will enable our team to study safety and tolerability of AST-005 while demonstrating that the SNA technology can be used to treat diseases locally using a nucleic acid therapy. We are excited to bring this approach to patients in need," said David Giljohann, CEO at Exicure, when the trial was first announced.
The results are looking promising. At the ASC meeting, one of the team, Chad Mirkin, explained that AST-005 has been found to be safe in humans, and shows a dose-dependent response to TNF-α.
This means that although there is more work to do in finding the correct dose, the researchers are hoping that a treatment could be on the way for those suffering psoriasis.
The researchers didn't go into much detail, as it is still very early days yet. The initial observations have only been discussed at the ASC meeting, there has been no paper published in a peer reviewed journal, so unfortunately we can't explain much more about the trial, or get too excited just yet.
But if the treatment continues to show promise in this and other follow-up trials, it's just the beginning for similar SNA therapies, with the potential for more new drugs based on this technique to target cancer-causing genes, and a number of auto-immune diseases.
Although this is just an initial observation, we're looking forward to seeing the final results.