Ageing is a natural part of being human, but for all that, we know very little about how it works - least of all in the most complex object in the known universe: the human brain.
Scientists at MIT are on a mission to better understand the mysteries of the ageing brain, and their newest findings are intriguing. The researchers have discovered an unusual sign of damage in the brains of mice and humans, and it could be an important marker for cognitive decline and disease.
The human brain churns through a spectacular amount of energy - far more than any other organ in the body - consuming 20 percent of all our daily oxygen needs.
But this voracious appetite comes with a hidden cost. As our brains burn through more and more energy, free radicals begin to build up in our noggins, and these byproducts are thought to damage some neurons.
This is called oxidative stress, and now, we might finally have a way to locate this damage in the brain.
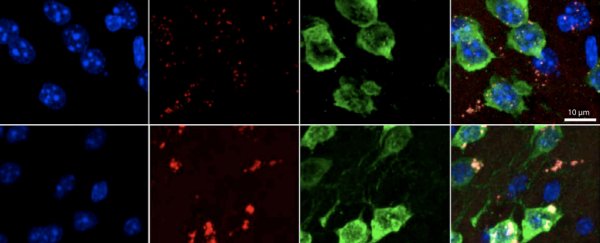
It all has to do with RNA. This is the messenger which carries instructions from our DNA that control protein production. In certain genes, however, the researchers found that RNA appears to be especially vulnerable to oxidative stress and this can cause it to behave in unusual ways.
The consequence is a build-up of RNA snippets in neurons - and it is easily identifiable if one uses the right technique.
"This phenomenon appears to be a previously unrecognised consequence of oxidative stress, which impacts hundreds of genes and may influence translation and RNA regulation globally," says co-author Christopher Burge, a researcher in genomics at MIT.
At first, Burge and his team compared two different types of neurons in the brains of mice: the spiny projection neurons, D1 and D2.
These neurons were chosen because together, they make up more than 95 percent of all the neurons in the striatum - a part of the brain associated with diseases like Parkinson's and Huntington's.
"These two types of neurons are implicated in several neurodegenerative diseases that are ageing-related, so it is important to understand how normal ageing changes their cellular and molecular properties," explains co-author Myriam Heiman, a researcher in cellular biology at MIT and Harvard.
Using green fluorescent tags, the researchers compared the D1 and D2 neurons in both 6-week-old mice and 2-year-old mice (which, in human year analog, is about 70 to 80 years old).
What they found was surprising: the D1 neurons appeared to be far more vulnerable to oxidative stress than the D2 neurons. In the older mice, the D1 neurons had around 400 genes that expressed only a short fragment of their original RNA sequence.
These short snippets, called 3′ untranslated regions (UTRs), appeared to be building up and sticking all over the ribosomes - a cell structure that builds proteins from RNA - stopping the proper production of protein.
When the researchers began looking at human brains, they noticed the same thing. There were piles of these RNA snippets in most parts of the human brain, including the frontal cortex - a particularly active region. Plus, the researchers found that as a brain aged, the amount of 3′ UTRs gradually increased.
The results were unexpected, but the authors are rolling with the punches. They propose that the whole thing rests on an enzyme, called ABCE1, that cuts away the ribosomes from the RNA after it is finished translating them.
The suspicion is that this enzyme is damaged by oxidative stress, which means it isn't as good at doing its job. So, instead of the ribosomes being cut off the RNA, they build up and stick to the exterior in greater and greater numbers, until a second enzyme comes along and cuts up the RNA into 3′ UTRs.
"Sending neural signals takes a lot of energy," Burge explains.
"Over time, that causes oxidative damage, and in our model one of the proteins that eventually gets damaged is ABCE1, and that triggers the production of 3′ UTRs."
Given the right mix of genetic and environmental factors, the authors think this type of cellular damage could possibly lead to cognitive decline and other neurodegenerative diseases.
As such, these little clumps of RNA could be an important way for scientists to identify which brains are more at risk from cognitive decline.
But before that becomes a reality, there's plenty more to research. We still don't know why 3′ UTRs accumulate in older brains or what the consequence of this might be.
Nevertheless, the new research is an important stepping stone as the world's ageing population continues to grow at an unprecedented rate.
This study has been published in Cell Reports.