Several weeks after conception, your body underwent a big step in development – you grew a top end and a bottom end. But how exactly our cells figure this out has been a pretty big mystery.
Now, by arranging a bundle of stem cells to act pretty much like an actual human embryo, researchers have gained a much better idea of the biochemistry behind how this process occurs in the developing human body.
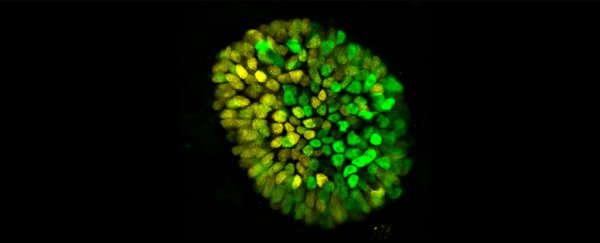
The diverse team of scientists from Rockefeller University combined backgrounds in physics and biology to encourage a model of a 10-day-old human embryo to change from a perfect sphere into something less symmetrical.
Restrictions on studying embryos that are older than 14 days currently make researching the earliest moments in our development a practical and ethical challenge. Unfortunately, animal models can only go so far when it comes to pulling apart the complex mix of genetic and biochemical activity that turns a fertilised cell into a fully formed human.
To get closer to defining the precise sequence of early changes required to mould 'blank slate' tissues into a human, researchers can take the same kind of nondescript stem cells that make up an embryo and arrange them to represent a key stage in development.
These models are actually more like embryonic organoids – synthetic structures that mimic some, but not all, of the defining characteristics of their biological counterparts.
These so-called embryoids have been used to study cell and embryological development for years, so technically aren't all that new as a laboratory tool.
But as synthetic approximations, finding the most appropriate structure to represent a specific developmental step isn't a straight-forward affair.
"We combined several techniques – bioengineering, physics, and developmental biology – to create this model," says physicist and biochemist Mijo Simunovic.
"We now have a 3D system that mimics not only the embryo's genetic fingerprint, but also its shape and size."
This specific 3D model had a very special job to do. Studies on mice embryoids have already revealed how a bland ball of dividing cells draws a line that distinguishes which end will grow a head and which will grow a tail - a moment known as symmetry breaking.
"Symmetry breaking drives almost everything that happens during embryonic development. Our heads don't look like our feet, and that's because, at some point, the embryo breaks into two parts, anterior and posterior," says Simunovic.
This important step, called gastrulation, occurs in all mammals. Two years ago researchers found evidence of the process in a ball of human embryonic stem cells, suggesting it might be possible to study in an embryoid designed for the task.
To detail the biochemistry behind gastrulation in humans, the team tested a growth factor that had already been found responsible for anatomical patterns in mice and human embryos called bone morphogenetic protein 4.
"We added BMP4, and two days later one part of the three-dimensional culture became the future posterior, and the opposite part became the future anterior," says Simunovic.
Not only do the findings help us to distinguish vital similarities and differences in experiments conducted on the embryos of other animals, they could also explain why some embryos are destined to develop, and some fail to implant at all.
The development of the embryoid model might be a big win for science, but innovation could eventually become its own worst enemy here.
The question of when a synthetic approximation of an embryo crosses the line between life-like model and authentic human becomes increasingly blurred as complexity grows.
In an interview with NPR's Rob Stein, the researchers emphasise there is a distinction, and while they are endeavouring to make more sophisticated models, their embryoids "would never become human embryos if we let them grow."
This research was published in Nature Cell Biology.