Perovskite has a lot going for it in our search for a cheap, efficient way to harvest solar energy. With a dusting of organic molecules, these crystalline structures have been able to convert more than a quarter of the light falling onto them into electricity.
Theoretically, perovskite crystals made with the right mix of materials could push this limit beyond 30 percent, outperforming silicon-based solar cells (which is currently the most abundant solar panel technology), and at a much lower cost. It's all good on paper, but in reality, something has been holding the technology back.
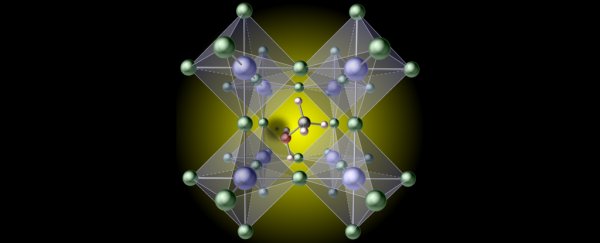
Combine calcium, titanium, and oxygen under the right conditions and you'll form repeating cages of molecules that look like a bunch of boxes joined at their corners.
Regardless of the elements involved, this particular crystalline pattern is called a perovskite structure. Make one from lead iodide, throw in an organic compound like methylammonium for a positive charge, splash on some sunshine, and you'll be on your way to generating a current of electricity.
To achieve efficiencies beyond 25 percent in this energy transformation, engineers quickly learned it pays to ensure there's plenty of iodide, seemingly to ensure any defects in the perovskite crystal lattice are well and truly filled.
But this assumption was never fully tested, so researchers from the University of California, Santa Barbara in the US went back to first principles to determine what was really going on.
Using cutting-edge computing to analyze the quantum behaviors influencing electrons as they migrate through the hybrid mix of organic molecules and lead iodide structures, the team discovered that adding more iodide wasn't exactly the wise move experiments suggested.
It turns out the flaw in the system wasn't where anybody had expected.
Rather than a flaw in the perovskite cages, it was the organic component – previously considered to be an unbreakable unit – that came with a rather frustrating weakness. It turns out their hydrogen can snap right off.
"Methylammonium lead iodide is the prototypical hybrid perovskite," says lead researcher and materials engineer, Xie Zhang.
"We found that it is surprisingly easy to break one of the bonds and remove a hydrogen atom on the methylammonium molecule."
That hydrogen vacancy forms a rather inconvenient pothole in the electrical highway, impeding the current generated as sunlight knocks electrons free from the surrounding perovskite structure.
"When these charges get caught at the vacancy, they can no longer do useful work, such as charging a battery or powering a motor, hence the loss in efficiency," says Zhang.
Though the process is entirely theoretical at this stage, the calculations also allow the team to find ways around the flaw.
One possibility, matched by experimental findings, would be to shoot for a middle ground in iodide concentrations.
Swapping out the organic molecule for another cation such as cesium, or better yet, a similar kind of organic compound like formamidinium, might also lead to a radical improvement in efficiency.
Transforming this theoretical work into a practical method of electricity generation will require a lot more testing and planning. What works in calculations would need to be woven into processes that grow flawless wafers of perovskite around molecules of formamidinium.
For perovskite to have a hope of dominating the energy production market, it will need to show its worth on both a financial and functional level.
Projections on silicon suggest there's still some way to go before it hits its theoretical limits beyond 30 percent.
But given the progress perovskite has made in just the past decade, perovskite solar cells could be due for their big break in the not too distant future.
This research was published in Nature Materials.