Scientists have uncovered changes in neurological structure that could underlie the autism spectrum disorder known as Pitt Hopkins syndrome, thanks to the help of lab-grown brains developed from human cells.
Furthermore, the researchers were able to recover lost genetic functions through the use of two different gene therapy strategies – hinting at the possibility of treatments that could one day give those with the condition new options in improving their quality of life.
Pitt Hopkins syndrome is a neurodevelopmental condition stemming from a mutation in a DNA-management gene called transcription factor 4 (TCF4). Classed on the autism spectrum on account of its severe impact on motor skills and sensory integration, it's a complex condition that presents with a range of severities.
What's more, changes in the TCF4 gene are associated with other forms of autism and diverse neurodevelopmental conditions, including schizophrenia.
In spite of its clear significance in our brain's development, we know surprisingly little about the gene's mechanisms, neither in its typical or mutated forms.
Researchers from the University of Campinas in Spain and the University of California San Diego (UC San Diego) aimed to change this by studying the workings of the genes in an environment as close to a developing brain as they could ethically get.
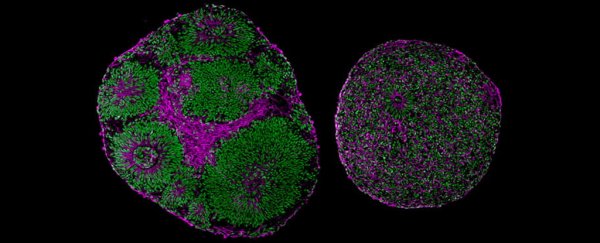
Skin cells taken from volunteers with a diagnosis of Pitt Hopkins syndrome were reprogrammed into stem cells that formed the foundations of a brain-like mass, called a brain cortical organoid.
Organoids are simplified versions of a real brain, incapable of performing all of the functions expected of an actual organ. Yet they do help researchers study aspects of the brain, demonstrating features like the order of tissue development, and the cascade of chemical triggers we might see in a growing fetus.
By studying the progress of tissues with the mutated versions of TCF4 taken from individuals with Pitt Hopkins syndrome, and comparing them against tissues with more typical TCF4 genes, the researchers could map changes in the tissues' structure and operation.
"Even without a microscope, you could tell which brain organoid had the mutation," says pediatrician Alysson R. Muotri from UC San Diego.
The masses created with atypical TCF4 genes were noticeably smaller than the control organoids, for one thing, with some showing a polarized distortion in their general structure.
The researchers also discovered that the version of the gene responsible for Pitt Hopkins syndrome freezes the progenitor cells that give rise to different types of neuron, impairing their ability to diversify.
This results in a reduction in the amount of neurons in the cortex, as well as a drop in their activity – two factors that could help explain the more profound differences in brains with autism or schizophrenia.
Part of the cause of this drop in neural differentiation seems to be a drop in a specific type of signaling that occurs across cell membranes.
By artificially supporting this signal through targeted pharmaceuticals, the researchers found they could return at least some of the neural diversity and electrical activity to the cortical areas of the organoids.
Genetically correcting the TCF4 mutations in the tissues also reversed the mutation's effects, making the organoids constructed from volunteers with Pitt Hopkins syndrome look more similar to control organoids.
"The fact that we can correct this one gene and the entire neural system reestablishes itself, even at a functional level, is amazing," says Muotri.
It's a small key piece of information that could one day lead to some revolutionary therapies, though that day is still a long way off.
Organoids aren't fully functional brains, leaving plenty of room for overlooked factors that could complicate matters.
More importantly, conditions such as autism and schizophrenia only become evident after birth. Without knowing how changes in the differentiation and activity of nerves impact the function of a more fully-formed brain, it's impossible to know the value of therapies like these.
But while it's a small step towards understanding how some neurodevelopmental disorders unfold, it's also a breakthrough that could give those affected by the mutated gene a choice in how they manage their wellbeing.
"For these children and their loved ones, any improvements in motor-cognitive function and quality of life would be worth the try," says Muotri.
This research was published in Nature Communications.