The life of a crested newt defies explanation from the very start.
Each year, once breeding season is done and dusted, each female lays a few hundred eggs, tucking every one individually into the leaves of pond plants to give them the best chance of a safe hatching.
Yet no matter how careful she is, half of her brood will never see the light of day.
When the Italian zoologist Mauro Rusconi first calculated this extreme death rate in crested newts (of the genus Triturus) in 1821, he was bewildered. Why would a female spend the time and energy to lay so many eggs that would never hatch? It didn't make evolutionary sense.
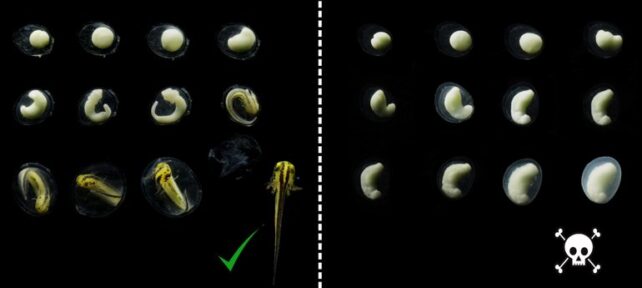
Unlike, say, a sea turtle, whose offspring sometimes have a one in ten thousand chance of making it to adulthood, the eggs of newts aren't even getting a chance to "struggle for existence", failing early in their development.
Why such a curse persists in every species of crested newt has long been a total mystery. In fact, for more than 150 years after Rusconi's initial observation, no scientist could figure out why so many newt embryos were doomed from the very start.
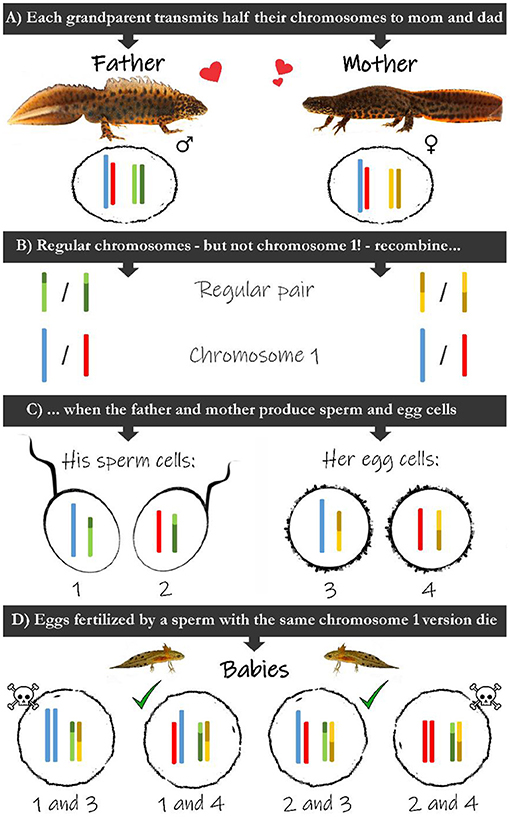
It took until 1980 for researchers Herbert Macgregor and Heather Horne to make a pivotal discovery, learning the 'cursed' newt embryos were dying from a weird genetic mutation in their number 1 chromosome, which made it longer. This long version – called 1A – has a mistake in it that isn't present in the other version, called 1B.
Surprisingly, 1B isn't exactly perfect either, with a different 'mistake' that renders critical information useless.

If a newt egg is fertilized by a sperm cell with the same variation of chromosome 1 (either 1A or 1B), it results in a deadly combination of 1A+1A or 1B+1B in the offspring. The other essential half is missing.
This isn't unusual in itself. Nature has a neat way of minimizing the odds of an embryo inheriting broken genes such as these, shuffling sections of chromosomes from each parent around with one another.
"Normally such errors can be fixed by recombination," researchers explain in an article from 2021, "chromosomes can swap the faulty DNA for functional DNA."
But in crested newts, chromosome 1 pairs aren't shuffled with one other, in spite of other pairs of chromosomes receiving a good mix-up. This means those with a deadly chromosome 1 pairing barely make it off the developmental starting block.
Those embryos that do survive will carry one 1A and one 1B chromosome, meaning that the next generation is doomed to undergo the exact same losses, over and over.
This scenario is known as a balanced lethal system, and it's one of the greatest mysteries in evolutionary biology.
So why hasn't natural selection eliminated a deadly inherited disease if its consequences are so deeply maladaptive?
Crested newts aren't the only animals that suffer from this strange, genetic kiss of death. Marbled newts and some insects, like fruit flies, also have balanced lethal systems, as well as a few plants.
"Explaining the evolution of balanced lethal systems requires a scenario in which short-term benefits 'fool' natural selection into opting for an arrangement that is actually detrimental in the long run," Leiden University evolutionary biologist Ben Wielstra explains in his 2020 paper on the topic.
Perhaps newts walked into this trap by accident. In other words, their deadly genes could be an evolutionary mistake or byproduct, inherited from a distant ancestor, that keep persisting because of a short term benefit.
Some scientists have hypothesized that a balanced lethal system evolved in newts from the 'ghosts of sex chromosomes past'. This describes a scenario where a newt's sex may once have been determined by its chromosomes, as it is in humans, but then evolved to be determined by temperature later on, as it is in many modern reptiles.
The problem with this hypothesis is that chromosome 1 plays no part in determining the sex of newts. That's the job of chromosome 4.
Besides, there are other animals that possess a balanced lethal system where temperature doesn't play a role in sex determination. So there must be another explanation.
Recently, a hypothesis that has become more popular looks to groups of genes inside chromosomes for answers.
Supergenes are genes that are lumped together and inherited as a whole unit, which means they cannot be recombined in offspring. If a recessive and lethal genetic mutation is attached to one of these supergene alleles, it could create a feedback loop whereby newts with 1A and 1B chromosome pairs are favored for survival and reproduction, keeping the mutations fixed in the population.
It's an intriguing idea, one that needs further, real world testing.
A recent study published in 2022 simulated this possibility and found that only when a population is tiny and a detrimental mutation is compensated for by the overall benefits of the supergene does a balanced lethal system appear in crested newts. And even then only sometimes.
At this evolutionary bottleneck, it seems newts could possibly enter a genetic trap, from which it is very hard to escape.
"All documented balanced lethal systems are supergenes but none of them has yet been studied at the genomic level," write researchers in the 2022 paper.
"With the advent of next-generation sequencing, properties associated with the evolution of balanced lethal systems can be investigated empirically."
After several centuries of head scratching, geneticists and evolutionary biologists finally have the tools they need to solve this enduring paradox.
