The sad reality of terminal lung illnesses is that there are simply far more patients than there are donor lungs available. This isn't just because of the low number of donors, which would be problem enough, but many donor lungs are significantly damaged, rendering them unusable.
By using a new experimental technique, though, such a damaged lung has now been restored to function - by sharing its circulatory system with that of a living pig. This leverages the body's self-repair mechanisms to exceed the capabilities of current donor lung restoration techniques.
"It is the provision of intrinsic biological repair mechanisms over long-enough periods of time that enabled us to recover severely damaged lungs that cannot otherwise be saved," say the lead researchers, surgeon Ahmed Hozain and biomedical engineer John O'Neill of Columbia University.
The underlying principle is similar to an existing donor lung restoration technique called ex vivo lung perfusion (EVLP), which involves placing a lung in a sterile dome attached to a ventilator, pump, and filters.
The lung's temperature is maintained at human body temperature, and a bloodless solution containing oxygen, nutrients, and protein is circulated through it. That circulation, when liquid is pumped through the organ, is the perfusion part.
EVLP has helped save lives by keeping donor lungs stable and even repairing them a little. But the time window afforded by the technique is somewhat limited - it can only be conducted for up to eight hours, which is not a lot of time for the biological repair functions to kick in.
It's that precious time that the research team has bought with their pigs, and years of research.
In 2017, O'Neill led the development of the xenogeneic (cross-species) cross-circulation platform. Last year, two of the researchers, biomedical engineer Gordana Vunjak-Novakovic of Columbia University and surgeon Matthew Bacchetta of the Vanderbilt Lung Institute, led a study in which they restored damaged pig lungs by attaching them to other pigs.
Earlier this year, the team extended the operation time of the platform to four days.
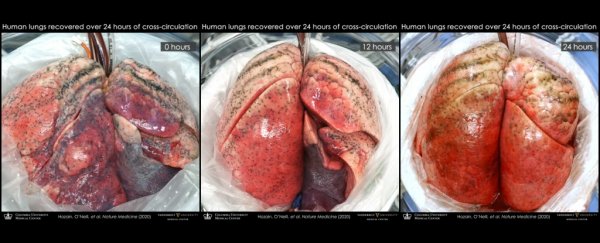
Now, the researchers have revealed that they have successfully used the same technique to repair five damaged human lungs by connecting them to pigs, including one severely injured lung that had failed to recover function using EVLP.
"We were able to recover a donor lung that failed to recover on the clinical ex vivo lung perfusion system, which is the current standard of care," Vunjak-Novakovic said. "This was the most rigorous validation of our cross-circulation platform to date, showing great promise for its clinical utility."
In the study, six donor lungs were obtained by the team after being rejected for transplantation. The five lungs in the experiment were attached via a jugular cannula to anaesthetised pigs that had been immunosuppressed, to prevent the pig's immune system from attacking the lungs. The sixth control lung was attached to a pig that was not immunosuppressed.
All of the lungs were subjected to 24 hours of xenogeneic cross-circulation, while the researchers carefully monitored the physiological and biochemical parameters of the organs.
The control lung did not take long to break down. It started developing extra fluid, the circulation broke down, while inflammatory and immune markers rose, blood clots formed, and respiratory function fell - all consistent with hyperacute rejection.
The contrast with the experimental lungs couldn't have been more remarkable. Even though all had previously demonstrated injuries, the lungs showed significant improvement in cellular viability, tissue quality, inflammatory response, and respiratory function.
The change in the lung that had failed EVLP in particular underwent a jaw-dropping turnaround. It had spent a total of 22.5 hours on ice, and received 5 hours of EVLP. After this, the right lung was accepted for transplant, but the left lung was simply too damaged. It had persistent swelling and fluid build-up. Multiple transplant centres had turned it down.
After 24 hours sharing blood with a pig, though, the damaged lung started to show signs of repair; not full recovery, but a lot more than had been thought possible. This suggests, the researchers said, that their cross-circulation platform could be used in conjunction with EVLP to help restore lungs that EVLP can't save alone.
It's not quite ready for clinical use, though. For one, the pigs could share things other than their blood. Like disease, for instance.
Because of this, any clinical use of the technique would require medical-grade animals, which would not be cheap - but it's nonetheless something that is under investigation for use in xenotransplantation, in which pigs' organs can be transplanted in human recipients. (This is currently being tested in baboons.)
The other option is that the human recipients themselves could potentially become the basis for the cross-circulation platform, being attached to the lungs they will themselves receive, and maybe even other kinds of organs one day.
"Modifications to the xenogeneic cross-circulation circuit could enable investigation and recovery of other human organs, including livers, hearts, kidneys and limbs," the researchers wrote in their paper.
"Ultimately, we envision that xenogeneic cross-circulation could be utilised as both a translational research platform to augment transplantation research and as a biomedical technology to help address the organ shortage by enabling the recovery of previously unsalvageable donor organs."
The research has been published in Nature Medicine.