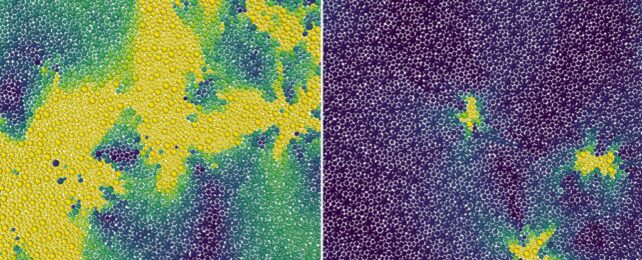
Glass might look and feel like a perfectly ordered solid, but up close its chaotic arrangement of particles more closely resemble the tumultuous mess of a freefalling liquid frozen in time.
Known as amorphous solids, materials in this state defy easy explanation. New research involving computation and simulation is yielding clues. In particular, it suggests that, somewhere in between liquid and solid states is a kind of rearrangement we didn't know existed.
According to scientists Dimitrios Fraggedakis, Muhammad Hasyim, and Kranthi Mandadapu of the University of California, Berkeley, there is a behavior on the temperature boundary of supercooled liquids and solids where the static particles remain excited, 'twitching' in place.
We're largely familiar with three fundamental states of matter in everyday life: solid, liquid, and gas, or vapor. Each is defined by the relationships between their particles and surrounds.
When one of these changes into another one – a solid melting into a liquid, or a liquid evaporating into a gas, for instance – this is known as a state transition.
But matter is quite a bit more complex than just those three basic states. Atoms can become so hot, for example, their charges fly apart to form a plasma. Cooled right down, some classes of particle can lose their identity altogether to blend into a quantum blur.
Amorphous solids are strange mixes of well-ordered solids and loosely-bound liquids. Where particles within solids tend to form predictable connections with their neighbors once they lock into place at suitably low temperatures, amorphic solids have the disordered arrangement of a liquid.
Just how these seemingly haphazard connections switch from viscous streams of flowing molecules to a static landscape is far from obvious.
Using glass as the most familiar example, its constituent elements of oxygen and silicon flow when heated. Cooled slowly, those particles have time to form into an ordered crystal structure called quartz. If it cools quickly, the particles somehow retain a disordered arrangement; this is the point at which it becomes an amorphous solid, and the temperature at which it occurs is the onset temperature.
Fraggedakis, Hasyim, and Mandadapu used computation and simulation, combined with the results of past experiments, to determine that this transition might not be so neat, featuring a special activity of particles sitting between their normal liquid and supercooled states.
"Our theory predicts the onset temperature measured in model systems and explains why the behavior of supercooled liquids around that temperature is reminiscent of solids even though their structure is the same as that of the liquid," Mandadapu explains.
"The onset temperature for glassy dynamics is like a melting temperature that 'melts' a supercooled liquid into a liquid. This should be relevant for all supercooled liquids or glassy systems."
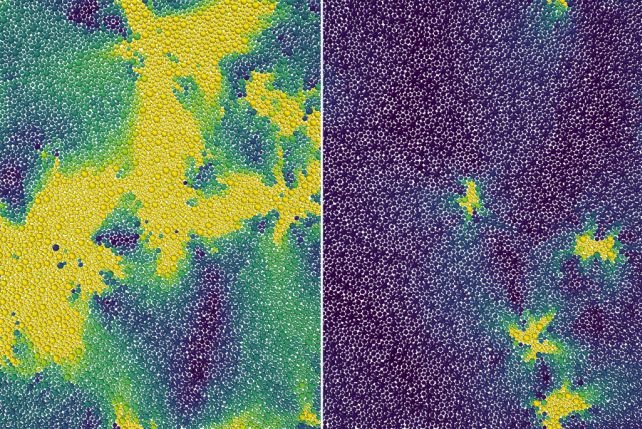
Although the overall flow of atoms in a supercooled liquid is effectively zilch, the particles are continuously changing their configurations while stuck in place, resulting in movements called excitations. The researchers treated these excitations in a 2D supercooled liquid as defects in a crystalline solid, and calculated what happens as the temperature changes.
They found that bound pairs of excitations become unbound at the onset temperature, causing the material to lose its rigidity and behave as a normal liquid.
The team believes that their model can be expanded to understand how the transition works in three dimensions, too, and offer a theoretical underpinning for future experimental work.
"The whole quest is to understand microscopically what separates the supercooled liquid and a high temperature liquid," Mandadapu says.
"It's fascinating from a basic science point of view to examine why these supercooled liquids exhibit remarkably different dynamics than the regular liquids that we know."
The research has been published in the Proceedings of the National Academy of Sciences.