The world is full of birds – chirping, fluttering, waddling; feathered, beaked, and clawed. These wonderfully diverse animals, the closest living descendants of the magnificent dinosaurs, have successfully made their homes on every corner of the globe.
But part of their reproductive process is still somewhat mysterious: the time their developing chicks spend hidden inside opaque eggshells, developing from a zygote to a hatchling that finishes its growth to adulthood tended by doting parents.
Now, a long-sought goal of avian developmental biology has been realized. A team of scientists has developed a system for culturing eggs that replaces the opaque eggshell of domestic white leghorn chickens (Gallus domesticus), allowing the observation of the bird's embryonic development from almost go to whoa.
So successful was the team's shell-less culture system that several chicks hatched successfully at the end of the normal incubation period and grew into normal chickens.
Scientists have been working for decades to try and develop a shell-less culture system, or SLCS, to observe the development of an embryonic bird. A previous method that showed promise, first described in 2014, involved allowing embryos to develop inside an egg for three days, which is required for the embryo's survival, then removing them to a culture vessel protected by a sheet of polymethylpentene kitchen cling film.

This system, however, was not perfect: the chicks were unable to develop normally, greatly reducing the hatch success rate.
A pioneer of that method, veterinary scientist Katsuya Obara of Okayama University of Science, along with his colleague Chizuka Obara, has led a team that solved one of the problems hindering normal development and hatching.
The yolk vitelline membrane protecting the layer of cells around the periphery of the embryo known as the blastoderm was drying out in the culture vessel, which the team thought could have been impeding normal embryonic development.
So, to prevent the membrane from drying out, the team mounted their culture vessel on a rotary shaker used to generate continuous motion, usually for mixing. The shaker's top plate was set to an angle of 7 degrees, and the experiment was tested with different periods of 6 rotations per minute, 10 rotations per minute, and 28 rotations per minute.
This prevented the membrane from drying out with a constant flow of albumen, but development and survival up to 10 days varied.
At 6 rotations per minute, the survival rate was highest, but all embryos showed signs of developmental delays.
At 10 rotations per minute, the survival rate was slightly lower, and some embryos developed abnormalities, but the rate of survival for normal embryos was strong.
And, at 28 rotations per minute, all of the embryos had non-survivable abnormalities by the 10-day mark.
With 10 rotations per minute the optimum rate for both survival and development, the researchers experimented by adding oxygen to the developing embryos at several different points past the 10-day mark. Although oxygen does need to be added, the timing at which this takes place doesn't really impact embryo development.
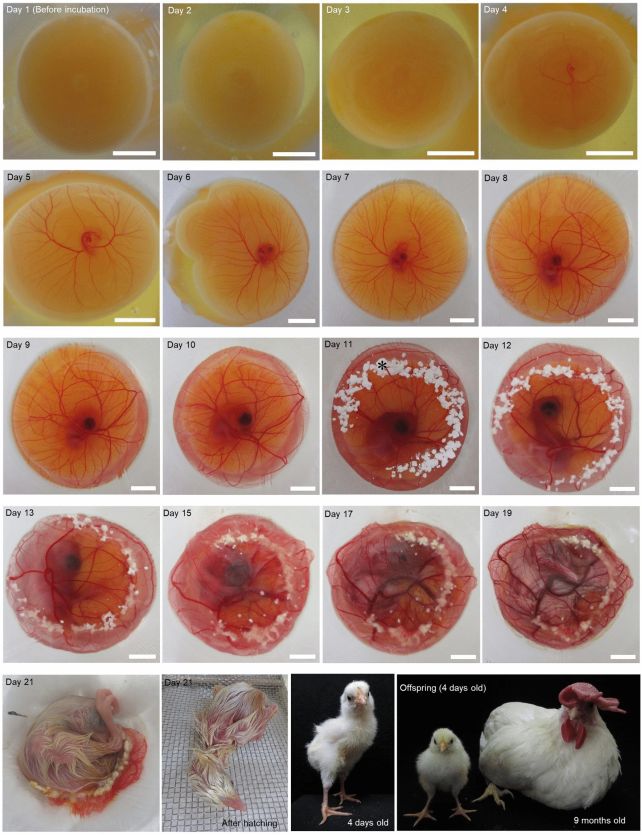
Finally, they conducted the same experiment – 10 rotations per minute, with the addition of supplemental oxygen, and a sprinkling of calcium carbonate powder (the material that eggshells are made of), but this time they implemented additional wiggling of the culture vessel by hand. This was performed three to five times every 3 to 10 hours in the first 24 to 48 hours of shell-less culture.
This method produced the highest hatch rate for normal chicks. At 10 rotations per minute, with oxygen, the hatch rate was 3.3 percent. Adding shaking back-and-forth by hand increased the rate to 10.5 percent.
One of the chickens that hatched was left to grow for a year, then euthanized and dissected. It was completely normal. The system is successful: it's given us a literal window to observe chicken development right up until hatching.
"The development of visible-cSLCSs has opened new possibilities by facilitating omnidirectional real-time monitoring of the phenotypic outcomes of experimental treatments through a transparent plastic film from blastoderm stage embryos until hatching," the researchers write in their paper.
"This system will serve as a powerful tool in various disciplines, such as toxicology, stem cell research, bioimaging, and regenerative medicine."
The team's findings have been published in Scientific Reports.
