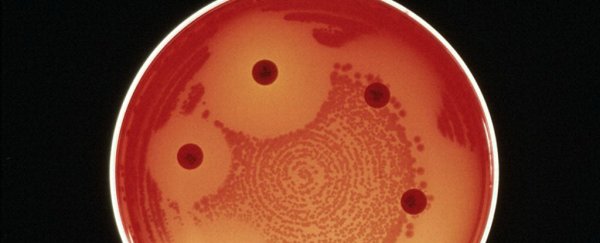
A woman in Nevada died in September from an infection that resisted every kind of antibiotic in the US.
The incident, which the CDC reported Thursday, is part of the growing problem of antibiotic resistance, which is on track to kill 10 million people by 2050.
"I think it's concerning. We have relied for so long on just newer and newer antibiotics. But obviously the bugs can often [develop resistance] faster than we can make new ones," Alexander Kallen, a medical officer at the Centres for Disease Control and Prevention's division of health care quality promotion told Stat News.
That's in part because it takes a long time to develop antibiotics, and even those that have made it through development face stumbling blocks.
As an unfortunate result, many major pharmaceutical companies have stopped developing new antibiotics altogether. Last year for example, the FDA turned down Cempra Pharmaceuticals' new antibiotic, a drug designed to fight a type of bacterial pneumonia called solithromycin, citing too little information on how the drug might impact the liver.
That additional trial would require testing out the antibiotic on 9,000 people.
Why it's so hard to get new antibiotics approved
Despite these roadblocks, biotech company Paratek Pharmaceuticals is currently working on a new antibiotic called omadacycline. So far, the approval process for the drug has taken roughly two decades.
The drug would treat skin infections, pneumonia, and urinary tract infections, and the company expects results from phase 3 trials looking at skin infections and pneumonia by July 2017.
"After 21 years of investment … we will have the pivotal data," Paratek President Evan Loh told Business Insider at the JPMorgan healthcare conference on Thursday.
From the time the drug went into human trials to when it could theoretically get on the market, 15 years will have passed, he said.
So why does it take so long?
Part of the problem is just tricky science: sorting through different compounds to figure out what antibiotic might work can take time. But it also has a lot to do with the companies running the trials staying afloat financially, said Loh. And at that point, sometimes legislation can come in handy.
Loh said the GAIN Act helped Paratek "save the company" by extending Paratek's patents on its antibiotic by five years. The act, which passed in 2012, aimed to incentivise companies to develop antibiotics by giving them extra time under patent protection to make money before facing generic competition.
If approved, Paratek's new drug could be added to the arsenal of medicines designed to take on resistant bacteria, which will be key as more deaths attributed to antibiotic-resistant bacteria occur.
"In the pre-antibiotic era, people were dead by the time they were 30 because of infections. Can you ever imagine that scenario where we get back to that situation?" Paratek Chief Commercial Officer Adam Woodrow asked Business Insider.
"There was once this time where we were keeping up. Now we've sort of fallen behind," said Woodrow. "And the group of antibiotics that should be there to combat these pathogens have just disappeared."
This article was originally published by Business Insider.
More from Business Insider: