New tactics in controlling infection are sorely needed, with antibiotic-resistant bacteria expected to claim as many as 2 million lives each year by 2050.
US and Spanish researchers have now discovered at least some bacteria pay a steep price for their resistance – a cost that we may be able to exploit to fight infection.
"We discovered an Achilles heel of antibiotic-resistant bacteria," says molecular biologist Gürol Süel from the University of California, San Diego.
"We can take advantage of this cost to suppress the establishment of antibiotic resistance without drugs or harmful chemicals."
Exploring why bacteria with resistance factors don't necessarily dominate their non-resistant relatives, University of California, San Diego biologist Eun Chae Moon and colleagues discovered an example of protection that comes at a cost, impeding the bacteria's ability to survive when levels of magnesium are low.
"While we often think of antibiotic resistance as a major benefit for bacteria to survive, we found that the ability to cope with magnesium limitation in their environment is more important for bacterial proliferation," Süel explains.
Depriving environments of magnesium could counter the bacteria's ability to thrive. And because unmutated strains don't share the same flaw, reducing the key nutrient shouldn't adversely impact bacteria needed for a healthy microbiome.
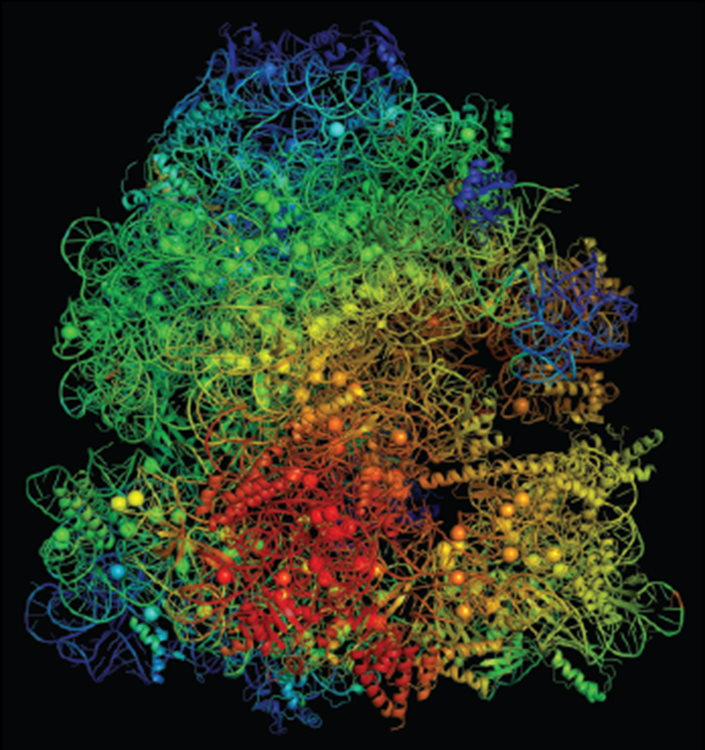
Charged metals like magnesium ions stabilize ribosomes, the micro machines in cells that create proteins. The ions also play an important role in the use of ATP that powers our cells.
A mutant version of the L22 ribosome in some Bacillus subtilis both protects the strain against antibiotics and tightly binds to the charged magnesium atom, leaving less for ATP to use for producing cellular energy. Moon and team's modeling revealed this physiological toll impedes the mutated strain's ability to grow and spread, compared to unmutated B. subtilis.
"Intracellular competition for a finite magnesium pool can thus suppress the establishment of an antibiotic-resistant ribosome variant," the researchers write in their paper.
This means that without the pressure of antibiotics, unmutated B. subtilis is fitter than antibiotic resistant B. subtilis.
"We show that through a better understanding of the molecular and physiological properties of antibiotic-resistant bacteria, we can find novel ways to control them without the use of drugs," Süel explains.
A limited comparison revealed that not all mutated ribosome variants have this weakness, so the researchers are keen to explore similar mechanisms in other bacteria as well.
"We hope that our work can help identify conditions that hinder antibiotic-resistant strains without requiring development of new antibiotics," Moon and team conclude.
This research was published in Science Advances.