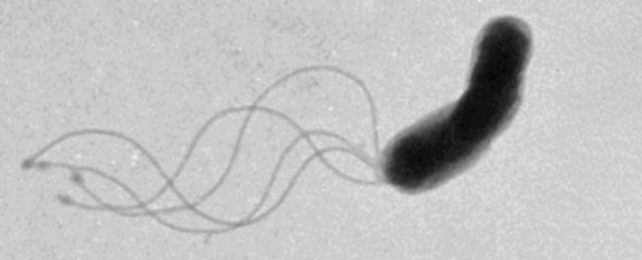
By peering through a high-tech microscope at flash-frozen proteins, researchers have just solved a 50-year-old mystery of how bacteria, and their ancient foe, archaea, actually swim.
We have long known they use a little coiled 'tail' called a flagellum, but details of how their stringy appendage forms its curled shape to push them forth has eluded our understanding – until now.
In animal cells, flagella work like the tails we're more familiar with – beating back and forth to propel their bodies forward. But cells belonging to bacteria, and the third domain of life, single-cellular archaea, have corkscrew-shaped flagella that can not generate thrust by simple side-to-side movement.
Instead, these little coils rotate like a twisted spindly propeller. Its coils appear to be able to stretch and contract to some extent, similar to a slinky, allowing the microbes to create different waveforms with their motor-driven rotations. The rotations can also change directions.
Both bacteria and archaea flagella are all composed of the same repeating subunits of flagellin protein. However, the type of flagellin found in the archaea tail is more similar to that found in another type of cellular protrusion found in bacteria called pili.
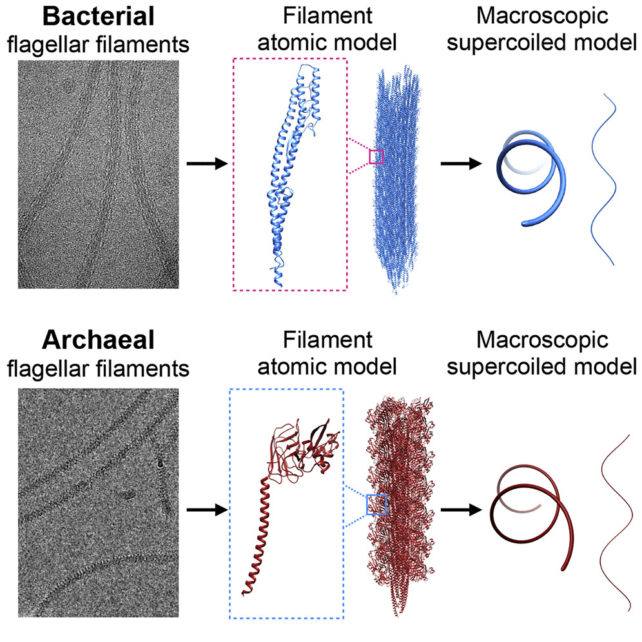
University of Virginia biophysicist Mark Kreutzberger and colleagues used cryo-electron tomography to examine the molecular structure of the flagellar filaments at a near-atomic level in the rod-shaped bacteria Escherichia coli and archaea Saccharolobus islandicus.
They saw that in the bacteria, the protein filaments could exist in 11 different states, and 10 different states in the archaea. It's having a mix of these states that causes the structure as a whole to curl into its coiled shape in both microbes, despite differences in protein structure.
The resulting supercoiled structure is so stable it can withstand torsion stresses, retaining its curled shape while being spun – that is, until the flagellum changes spin direction.
In E. coli, straight swimming involves counter-clockwise rotation. But when the bacteria switch the spin direction of their tail, the forces imposed on the flagella alter its structure, screwing one or more of their filaments out of their tight bundling and loosening the supercoils into a semi-coiled or curly shape.
This changes the microbe's straight swimming mode into a tumble with the tail's now clockwise spin.
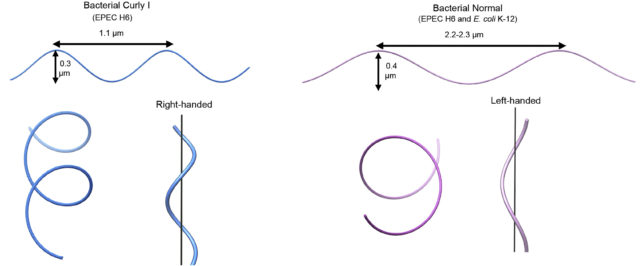
These direction-induced changes were not seen in the archaea, although changing their environmental conditions by adding salt or acid did alter their flagella's structure.
Despite their differences in structure and that they evolved independently, nature has shaped both bacteria and archaea's flagella to essentially have the same form and function – a neat example of convergent evolution.
"As with birds, bats, and bees, which have all independently evolved wings for flying, the evolution of bacteria and archaea has converged on a similar solution for swimming in both," explains University of Virginia biochemist Edward Egelman.
"Our new understanding will help pave the way for technologies that could be based upon such miniature propellers."
This research was published in Cell.