It's a textbook moment centuries in the making: more than 200 years after scientists started investigating how water molecules conduct electricity, a team has finally witnessed it happening first-hand.
It's no surprise that most naturally ocurring water conducts electricity incredibly well - that's a fact most of us have been taught since primary school. But despite how fundamental the process is, no one had been able to figure out how it actually happens on the atomic level.
"This fundamental process in chemistry and biology has eluded a firm explanation," said one of the team, Anne McCoy from the University of Washington. "And now we have the missing piece that gives us the bigger picture: how protons essentially 'move' through water."
The researchers, led by Yale University's Mark Johnson, were able to witness water molecules passing along protons - positively charged subatomic particles - using spectroscopy, a process that allows researchers to fire light at molecules and see what's happening inside.
Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't actually conduct electricity, because of its lack of free electrons.
But, in nature, pretty much all water has mixed with sediments and minerals, which ionises water molecules and allows them to conduct current.
Until now, all researchers really knew about that process was that H2O passes protons from molecule to molecule via their oxygen atom, sort of like a molecular relay race.
This process is called the Grotthuss mechanism, and was first described by chemist Theodor Grotthuss in 1806.
"The oxygen atoms don't need to move much at all," said Johnson. "It is kind of like Newton's cradle - the child's toy with a line of steel balls, each one suspended by a string. If you lift one ball so that it strikes the line, only the end ball moves away, leaving the others unperturbed."
You can see an illustration of the Grotthuss mechanism in the gif below:
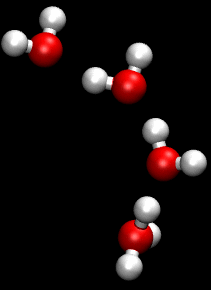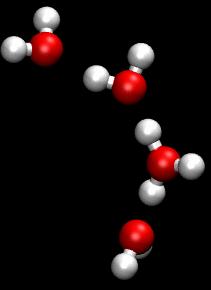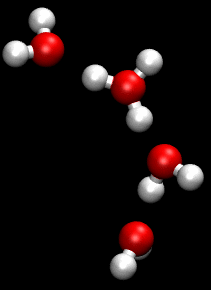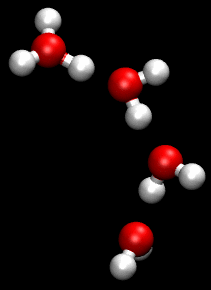 Matt K. Petersen
Matt K. Petersen
But until recently, this gif was about as detailed as our understanding went. Although researchers had a pretty good idea of how this mechanism worked on the surface, the details of exactly how that happened have remained frustratingly murky.
So for the past 200 years, researchers have been looking for an experimental way to follow the structural changes in water molecules as they conduct electricity - something that's proved incredibly challenging.
In recent years, researchers have tried to do this by using infrared scanning to monitor the progress, but the results came out looking like a blurry photograph, with no discernible detail.
"In fact, it appeared that this blurring would be too severe to ever allow a compelling connection between colour and structure," explained Johnson.
To figure it out once and for all, Johnson and his team found a way to fast-freeze the chemical process, so that snapshot moments in the process can be isolated and frozen in time, allowing them to get a closer look.
They used five molecules of 'heavy water' - water made from the deuterium isotope of hydrogen - and then chilled the molecules to close to absolute zero (–273.15 degrees Celsius or –459.67 degrees Fahrenheit).
When they did this, it slowed everything down, and suddenly the images of the protons in motion became a whole lot clearer.
"In essence, we uncovered a kind of Rosetta Stone that reveals the structural information encoded in colour," said Johnson. "We were able to reveal a sequence of concerted deformations, like the frames of a movie."
The new understanding will provide crucial insight into the conductivity of water - a phenomenon that keeps us alive, and is crucial to many chemical reactions on Earth.
But it could also help explain other mysteries out there, such as the long-standing debate over whether the surface of water is more or less acidic than the rest of its volume. This new imaging technique could answer that question once and for all.
It might also shed some light on some of the other recently discovered strange behaviours of water, such as the presence of a mysterious second liquid phase, and its weird ability to freeze solid at boiling point when confined to carbon nanotubes.
The team now wants to perform the experiments again with more water molecules, as well as other small molecules, to see how conductivity changes.
It might seem pointless peering so deeply into processes we already knew existed, but this type of fundamental research is the key to understanding the world around us.
After all, it's only when we truly know how matter behaves on the smallest level that we'll have a chance of figuring out the rest of the Universe. And water, despite how ubiquitous it is, is one of the weirdest molecules out there. The more you know…
The research has been published in Science.
