Researchers have reversed 'irreversible paralysis' in mice with complete spinal cord injuries using gene therapy.
The team found that regrowing neurons is not enough to fully restore walking and that regenerative therapies must target specific neurons and guide them back to where they belong, which was previously unknown.
There's a lot more work to do, but the Swiss and US team says it's the first step toward establishing the technology required to achieve the same in humans.
"We expect that our gene therapy will act synergistically with our other procedures involving electrical stimulation of the spinal cord," says neuroscientist Grégoire Courtine from the Swiss Federal Institute of Technology (EPFL).
A complete spinal cord injury (SCI) prevents the spinal cord from healing itself. Recovery requires inducing regrowth of neurons, which has been achieved, but the necessary conditions for these therapies to actually restore motor function remain a mystery.
We know that mice or humans with a partial SCI become temporarily paralyzed, but most of their motor function returns naturally. The spinal cord neurons that produce walking are located in the lumbar region, but after a partial SCI, neurons in the thoracic region relay communication across the spinal cord that allows walking to be restored.
The team hypothesized that restoring these specific neuron communication patterns could allow someone with a complete SCI to walk again, too.
"We were inspired by nature when we designed a therapeutic strategy that replicates the spinal-cord repair mechanisms occurring spontaneously after partial injuries," says EPFL neuroscientist Jordan Squair.
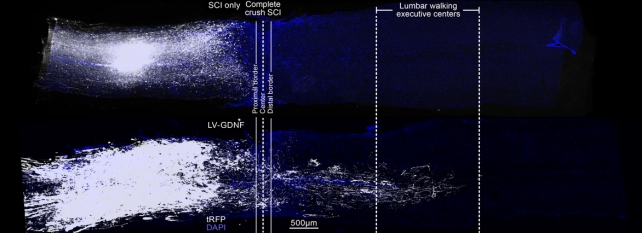
The researchers used genetic analysis to figure out which thoracic spinal cord neurons aid natural healing after a partial SCI. They followed their axons, the tiny nerve fibers that connect neurons and let them communicate, and found they naturally reach the lumbar spinal cord.
"Our observations using single-cell nuclear RNA sequencing not only exposed the specific axons that must regenerate, but also revealed that these axons must reconnect to their natural targets to restore motor function," says Squair.
In 2018, the same team demonstrated a treatment that stimulated the regeneration of axons in mice, which were able to grow back across severe damage to the spinal cord.
"But we also realized this wasn't enough to restore motor function," explains Mark Anderson, a neuroscientist at EPFL, "as the new fibers failed to connect to the right places on the other side of the lesion."
So the neuroscientists developed a gene therapy to stimulate regrowth of the axons and guide them all the way to the lumbar spinal cord.
They switched on growth programs in the identified neurons so that their axons could grow back. They also upregulated proteins that help the axons propel through the tissue core of the injury, and gave them guidance molecules to help them find their natural targets in the lumbar spinal cord.
This resulted in significant recovery in mice with complete SCIs. The mice were able to walk again, with walking patterns similar to mice that started walking again on their own after partial SCIs.
When they disabled these neurons, the mice could no longer walk, showing that this restoration of function was dependent on the regenerated axons of the specific neurons.
The authors say that figuring out how to lead specific subpopulations of neurons to their natural target regions is a step toward technology that can restore motor functions in humans.
"We believe a complete solution for treating spinal cord injury will require both approaches – gene therapy to regrow relevant nerve fibers, and spinal stimulation to maximize the ability of both these fibers and the spinal cord below the injury to produce movement," Courtine says.
The complexity of promoting regeneration over longer distances in larger animals like us means a successful strategy will likely require a lot more research.
The team concludes that their work will "unlock the framework to achieve meaningful repair of the injured spinal cord and may expedite repair after other forms of central nervous system injury and disease."
The study has been published in Science.
