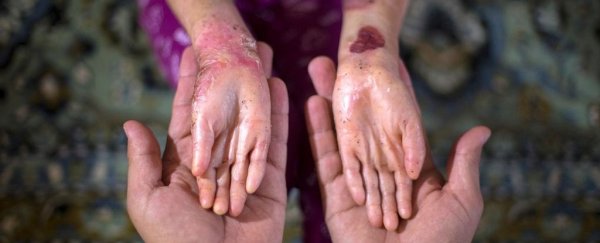
An incredible new gel could help close the blistering wounds associated with a rare skin disease known as epidermolysis bullosa (EB).
EB is an inherited condition that causes skin to be so fragile, it can tear at the slightest touch, like the wings of a butterfly.
While some mild forms of the disease can improve with age, there is no cure for severe cases, which can ultimately be fatal.
Stem cell treatments have proven effective for some so-called 'butterfly children', but this invasive technique requires skin grafting, which is expensive and necessitates anesthesia and hospitalization.
Researchers at Stanford University have now come up with a cheaper and easier treatment that could bring longed-for relief to so many affected by the condition.
The topical gel can be applied during regular wound dressing and contains a therapeutic gene that is spread directly onto the skin. The treatment works by replacing the gene that encodes for collagen VII (C7), which is a protein missing in those with recessive dystrophic epidermolysis bullosa (RDEB).
This particular collagen anchors the foundational structure of human skin, holding the dermis and epidermis together.
Without C7, patients with RDEB spend their lives tackling painful blisters, scarring, and sometimes even skin cancers.
Instead of grafting new skin onto these patients, the gel treatment 'injects' genetically modified fibroblasts directly into the skin, and it does so via the cold sore virus, herpes simplex type 1 (HSV-1).
HSV-1 is capable of infecting skin cells and can evade our immune systems. After genetically engineering the virus so it cannot replicate and spread to other parts of the body, researchers used HSV-1 to carry two genetic variants that code for C7 into the skin.
Once it's in the skin, clinical trials showed the topical treatment promotes skin integrity and robust C7 expression.
"Taken together, we demonstrate here a novel, easy-to-administer, and highly accessible gene therapy capable of reversing genetic disease through repeated application directly to patient skin wounds," the authors write in a new study.
If used early enough in the wounding process, the gel has the potential to halt further skin-tearing and scarring, thereby reducing the risk of skin cancer development and prolonging the lives of patients.
Other researchers have tried to make similar gels, but this is the first to pass rigorous clinical testing with flying colors.
When applied to the wounds of nine RDEB patients over the age of six, the gel showed remarkable results in randomized, placebo-controlled trials.
After three months and three doses, all the wounds that received the gel had healed and closed. A few weeks after that, the wounds still remained closed.
Meanwhile, the placebo-treated wounds continued to heal and then re-blister in a vicious and painful cycle.
Only two wounds in the trial did not fully heal after three months of gel application. One wound had been bothering a patient for about five years, but after a second three-month treatment cycle, the lesion closed and remained healed for eight months.
Another wound that had persisted for four years and covered most of a patient's side healed by 70 percent with the help of the gel.
Biopsies of all the patients revealed that the gel began triggering collagen production as early as nine days into the trial.
Even better, there were no severe adverse side effects triggered by the ointment, and it worked similarly for all age groups and genders.
"The wounds heal quickly but, even more importantly, they stay closed," says dermatologist Peter Marinkovich from Stanford University.
"The therapy strengthens the skin and breaks the painful and destructive cycle of wound opening and closing that patients with epidermolysis bullosa experience."
The phase III clinical trials are already complete, and while the results have not yet been formally published, the company that funded the research, Krystal Biotech, has shared some of the initial results.
Of all 31 patients enrolled in the trial, 67 percent of the wounds treated with the gel for six months showed complete healing. In the same timeframe, the placebo healed only 22 percent of the wounds.
"We saw no problems with repeated administration of the gel, and the patients and their families were very enthusiastic about the results," says Marinkovich.
"I'm excited that, if approved for clinical use by the Food and Drug Administration, we will be able to reach many more patients with this devastating disease."
The study was published in Nature Medicine.