Thanks in part to the atom's size, a charged variation of gold called Au2+ isn't commonly found in nature.
Scientists from Stanford University, the University of California, Berkeley, and McGill University in Canada have now managed to create and stabilize the rare ion, allowing for a host of new uses for this fascinating elemental metal.
Gold atoms have a lot of protons packed into their nuclei, giving them a large positive charge that tugs hard on their orbiting electrons. These unusually strong forces make the effects special relativity on their acceleration far more significant, making some arrangements of electrons more likely than others.
Not only does this relativistic configuration give gold its yellowish luster, it makes the loss of one or three electrons more likely than the loss of two.
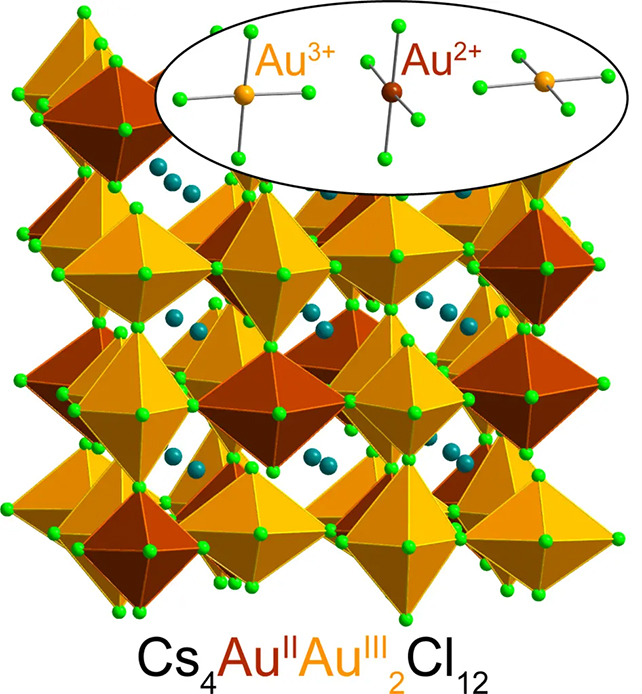
Here, the chemists trapped gold ions in a type of crystalline material called a halide perovskite, which is used everywhere from solar panels to electronic devices.
The special configuration of elements in the crystal stabilized any Au2+ ions that formed within its matrix, effectively preserving their unique state.
"Halide perovskites possess really attractive properties for many everyday applications, so we've been looking to expand this family of materials," says chemist Kurt Lindquist, previously at Stanford University and now at Princeton University.
"An unprecedented Au2+ perovskite could open some intriguing new avenues."
Discovered as part of separate research into magnetic semiconductors and how they might improve electronic devices, the formula includes a mix of cesium chloride salt, Au3+ chloride, water, hydrochloric acid, and "a little vitamin C".
"In the lab, we can make this material using very simple ingredients in about five minutes at room temperature," says Lindquist.
"We end up with a powder that's very dark green, nearly black, and is surprisingly heavy because of the gold it contains."
By donating one of its electrons, the vitamin C transforms the Au3+ ion into Au2+. Mumerous analyses confirmed the scientists had indeed produced this rarest of gold states in a stable form.
Gold is an attractive element for a lot of reasons, besides its rather eye-catching color: it's relatively easy to reshape, but doesn't easily react with other chemicals, meaning it doesn't tarnish or degrade over time.
Now we have a new, stable form of it to make use of, the next steps are to look more closely at the optical and electronic characteristics of Au2+, characteristics that could eventually be tweaked and used in electronics and other fields.
"It was a real surprise that we were able to synthesize a stable material containing Au2+ – I didn't even believe it at first," says chemist Hemamala Karunadasa from Stanford University.
"We're excited to explore what an Au2+ perovskite could do."
The research has been published in Nature Chemistry.