The microbial ecosystem nesting in your mouth is giving scientists a rare tool to learn about how bacteria multiply.
One of the most common bacteria living in your dental plaque, a filamentous bacterium called Corynebacterium matruchotii, divides not into two daughter cells like most cell divisions but multiple new microbes in a rarer process called multiple fission.
Led by microbiologist Scott Chimileski of the Marine Biological Laboratory in the US, a team of scientists observed single C. matruchotii cells dividing up into up to 14 new cells – a feat that can tell us how these organisms form the scaffolding that supports the hosts of other microbes that are dwelling in your mouth.
"Reefs have coral, forests have trees, and the dental plaque in our mouths has Corynebacterium," explains microbiologist Jessica Mark Welch of ADA Forsyth Institute and the Marine Biological Laboratory.
"The Corynebacterium cells in dental plaque are like a big, bushy tree in the forest; they create a spatial structure that provides the habitat for many other species of bacteria around them."
Most bacteria and archaea reproduce via an asexual process called binary fission. The genetic material divides, and the cell itself then divides, resulting in two organisms where there was one.
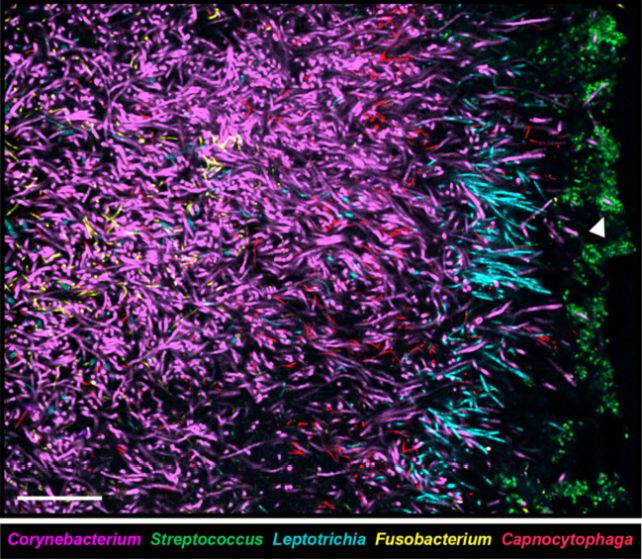
Welch and her colleagues became interested in the way C. matruchotii propagates after conducting a previous study into the way colonies of plaque bacteria organize themselves spatially in the biofilm that coats human teeth. The plaque microbiome forms a sort of spiky 'hedgehog' structure, with filaments of C. matruchotii as a base.
To observe how these structures add new filaments, the researchers used time-lapse microscopy, observing in real-time how the bacteria within the microbiome interact with each other, coexist, propagate, and grow.
This is where they saw the unusual cell division of C. matruchotii was not the normal binary kind, but much more prolific. And it does so in a very strange way.
First, the filament elongates at just one end, growing much longer than the usual size of the cell. It does so at a rate five times faster than other, closely related Corynebacterium species that live in the nose or on the skin.
Then, a number of dividing walls called septa form simultaneously, before the cell breaks apart into between 3 and 14 complete daughter cells.
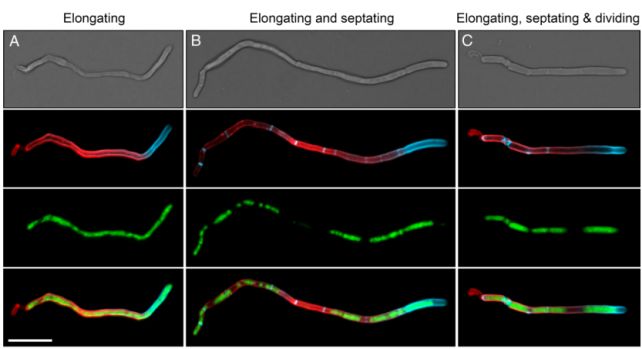
Thanks to this strange process, a colony of C. matruchotii can grow very fast indeed, up to half a millimeter per day – which might help explain why plaque starts to return to your teeth within hours, no matter how strenuously you clean them.
"These biofilms are like microscopic rainforests. The bacteria in these biofilms interact as they grow and divide. We think that the unusual C. matruchotii cell cycle enables this species to form these very dense networks at the core of the biofilm," Chimileski says.
"Something about this very dense, competitive habitat of the dental plaque may have driven the evolution of this way of growing."
Another interesting thing about C. matruchotii that might drive its strange growth and division is that it lacks a flagellum; the whip-like appendage other bacteria use to get around. Because it is fixed in place, its fast growth could be a means of exploring its environment and looking for sources of food, the researchers say.
It could be how the microbe gains a competitive advantage in the bacteria-crowded environment of the human mouth. But we've never seen anything quite like it. It's an entirely new way for bacteria to thrive – and it's been right here, all along, in our own bodies.
"We propose that rapid growth by tip extension and simultaneous multiple division explain how C. matruchotii outcompetes other taxa to form filamentous networks at the core of the dental plaque biofilm," the researchers write in their paper.
"Our findings extend beyond the oral microbiome, revealing a unique bacterial cell cycle and an example of how cell morphology and reproductive strategy can influence the spatial organization of microbial communities."
The findings have been published in the Proceedings of the National Academy of Sciences.
