Insects have their own suite of friendly microbes that help keep them healthy, just as we do.
But instead of simply growing larger like us sensible vertebrates, insects undergo extreme body warping to metamorphosize into their older stages of life.
As you might expect, these life-changing contortions complicate the microbes' living arrangements.
This convoluted process can completely distort and drastically shift organs and other tissues around, so any microbes along for the journey would possibly not be in for a survivable, let alone fun, ride.
Evolutionary ecologist Rebekka Janke from Johannes Gutenberg University of Mainz and colleagues took a close look at this transformative process in the darkling beetle Lagria villosa to find out what happens to their little helpers during this upheaval.
While darkling beetles have many symbiotic species within their microbiome, they particularly rely on Burkholderia bacteria for successful reproduction.
The beetle's eggs and larvae are vulnerable to infections, but Burkholderia, which female beetles ooze out from glands near their ovaries onto their eggs, keeps their offspring safe by producing polyketide chemicals that have antimicrobial properties.
As a consequence, one particular Burkholderia strain, B. gladioli, or Lv-StB, has become so accustomed to living a cushy life within the beetle that it has lost all ability to move of its own accord: Its genes and cellular structures for motility are almost completely gone, so it is reliant on the beetles for its own survival too.
Janke and the team tracked what happened to the microbes using fluorescent markers and microCT scans, and a sampling of the bacteria's DNA.
After the mother beetles coat their eggs with their bacteria-containing gland goo, the microbes spend around six days exposed on the eggs' surface, fighting off parasitic bacteria and hungry fungi.
Once the baby beetle grubs hatch, the bacteria gather into three deep lower back folds in the larva's outer cuticle, like back pockets. These folds don't just provide the symbiotes with protection; they also contain glandular cells that likely help nourish the bacteria with secretions.
But even these pockets get wrinkled during the extreme metamorphosis. They do, however, allow some of the bacteria to escape onto the surface, this time onto the pupae, ready to make their move towards the adult beetle's reproductive organs.
The researchers did not detect any B. gladioli in the pupae guts, so traveling to their hosts' glands is clearly not happening through internal paths.
So researchers put tiny symbiont-sized fluorescent beads of polystyrene onto the developing pupae. Most beads ended up around the tip of the beetles' abdomens once they emerged as adults, after their cases just happened to split open exactly where their back pockets were.
"By modifying unique 'pockets' on their backs, Lagria beetles manage to keep their protective symbionts and facilitate their relocation during pupation to newly developed adult organs," says evolutionary ecologist Laura Flórez of the University of Copenhagen.
The final stage of the bacteria's journey into the adult glands still remains a mystery, and most of this process only occurs in female beetles. The males start losing bacteria from the pupal stage – their back pockets are much smaller and shallower, and male adults lack symbiotes.
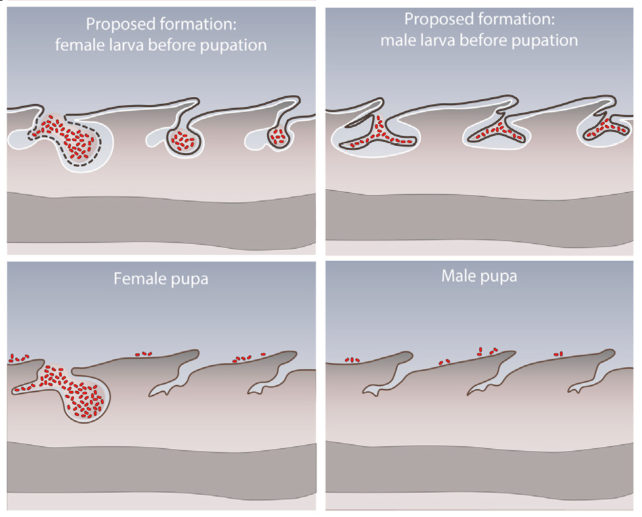
"In the adult stage, the main purpose of the symbiotic organs seems to be to enable successful transmission onto the egg stage and to the next generation," explains Flórez. "Since only females lay eggs, male adults do not need to carry these potentially costly symbionts and are a dead-end for the bacteria."
In social insects like ants, if individuals lose some of their microbial companions, they can be seeded back again from others in the group; the study's new results provide an example of how solitary insects avoid this potential loss during their most vulnerable life stages.
"These findings indicate that the ecological importance of the symbionts likely drove the evolution of specialized structures in the host to house and maintain the bacteria during metamorphosis," Janke and her colleagues conclude in their paper.
This research was published in Frontiers in Physiology.
