Scientists have thought for decades that one area of the brain simply disappears during human development.
Now, genetic similarities between cells in the subplate and neurons linked to autism suggest a different scenario.
In a new paper, researchers demonstrate that subplate neurons survive, and in fact become part of the adult cerebral cortex, a brain area involved in complex cognitive functions.
Researchers outline a connection between subplate neurons and certain brain disorders, and further identifies a strategy for treating such disorders via innovative stem cell techniques.
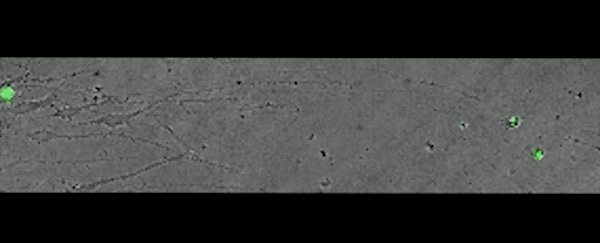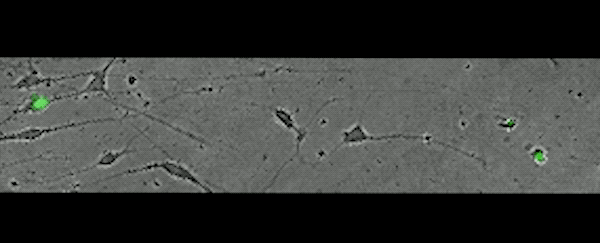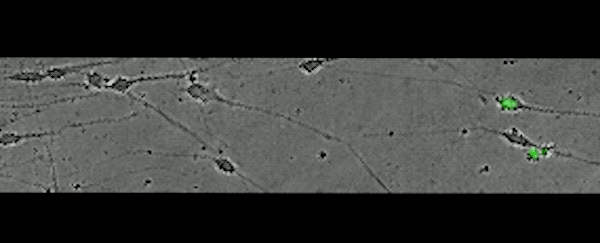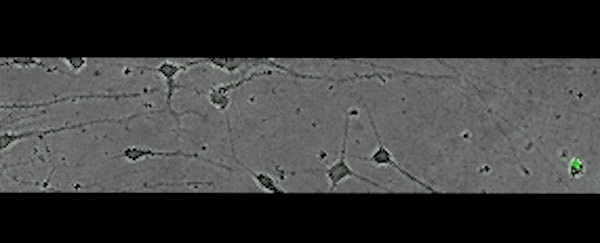
In the developing brain, the subplate (shown in green below) sits below the cortical plate, a precursor to the cortex. But in the adult brain it's curiously absent.
 Subplate is in green. (Rockefeller U)
Subplate is in green. (Rockefeller U)
During some stages of development, it's the largest layer of the brain — making its ultimate disappearance in the adult brain all the more confounding.
"When we think about cellular-replacement therapy, we need to think about how these cells are made in the first place. The understanding about the subplate was that it expands and then the cells of the subplate just die out," says Ali H. Brivanlou, professor at Rockefeller University.
"But we hypothesized: What if these subplate cells are not dying? What if they're just moving to a different level of the cortex—becoming part of the cortex?"
This movie, captured over 17 hours, shows subplate neurons migrating away from their original position — a clue that these cells don't die, but rather relocate.
 (Rockefeller U)
(Rockefeller U)
Brivanlou and colleagues found ample support for this idea. In samples of brain tissue from various developmental stages, they detected PRDM8, a protein expressed in migrating neurons that helps cells move into the cortical plate.
They also detected PRDM8 in subplate-like neurons that they generated from stem cells; and experiments showed that these laboratory-grown subplate neurons wandered away from their original location.
All of these findings pointed not to cell death, but to cell movement, they say.
Far from a site of demise, the subplate seems to nurture the development of functional and diverse cells. Brivanlou observed that subplate neurons mature into various types of deep projection neurons — cells found in the deepest layers of the cortex.
Preventing disorders
In other experiments, the researchers modulated the levels of WNT signaling, a pathway known to guide many developmental processes and found that the level of WNT signaling determined the fate of subplate neurons: low levels yielded projection neurons that extend within the cortex, and high levels yielded neurons that project to other brain areas, according to the study.
The findings, which appear in Cell Stem Cell, have significant implications for understanding brain disorders, the researchers say.
Projection neuron abnormalities have been linked to several neurodevelopmental conditions, including autism; and the new research suggests that these abnormalities manifest very early in development.
"A lot of the genes associated with autism are first expressed in the subplate," says postdoctoral associate Zeeshan Ozair. "And if subplate neurons don't die but instead become part of the cortex, they will carry those mutations with them."
In addition to shedding light on the early stages of brain disorders, the research presents new hope for preventing or treating such disorders through stem-cell therapy.
For example, the scientists hope that their findings will one day make it possible to treat neurodegenerative disease using techniques to generate scarce neuronal subtypes from subplate-like stem cells.
"The deep layers of the cortex are involved in many diseases: Alzheimer's, Lou Gehrig's, and Huntington's disease all kill off specific types of deep-projection neurons," says Ozair.
"When we think about cellular-replacement therapy, we need to think about how these cells are made in the first place."
"This research shows us how to generate these neurons directly, because we know the signaling mechanism that is necessary for their fate to be unveiled," Brivanlou says.
Source: Rockefeller University, Original Study
This article was first published on Futurity. Read the original article.