In a feat of modern-day alchemy, scientists have used a beam of vaporized titanium to create one of the heaviest elements on Earth – and they think this new method could pave the way to even heftier horizons.
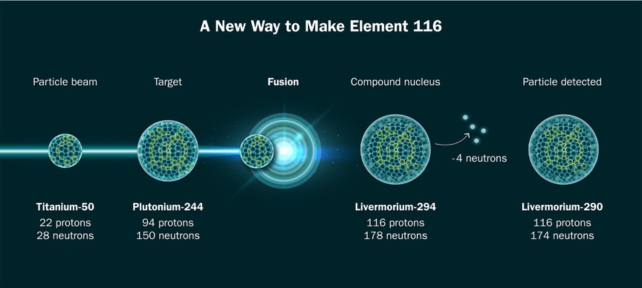
This is the first time the new technique – in which a hunk of the rare isotope titanium-50 is heated to almost 1650 °C (3000 °F) to release ions that are beamed at another element – has successfully produced a superheavy element, livermorium.
Livermorium was first synthesized back in 2000, and it's not the heaviest element humans have created (that would be oganesson, atomic number 118).
So what's the big deal if a couple atoms of livermorium recently popped into existence at the Lawrence Berkeley National Laboratory – those keeping track of the periodic table might ask? Livermorium is 'so Y2K', and has only 116 protons.
But fusing a titanium beam with plutonium to create livermorium is just a test run for much bigger (or rather, heavier) things. The scientists hope to create an element that will be the heaviest ever produced: unbinilium, with 120 protons.
"This reaction had never been demonstrated before, and it was essential to prove it was possible before embarking on our attempt to make 120," says nuclear chemist Jacklyn Gates of Berkeley Lab, who led the research.
Calcium-48, with its 20 protons, has been the go-to beam, because its 'magic number' of protons and neutrons makes it more stable, helping it fuse with its target.
Titanium-50 is not 'magic', but it has the 22 protons needed to reach those heavier atomic weights, without being so heavy that it simply falls apart.
"It was an important first step to try to make something a little bit easier than a new element to see how going from a calcium beam to a titanium beam changes the rate at which we produce these elements," physicist Jennifer Pore from Berkeley Lab explains.
"Creating element 116 with titanium validates that this method of production works and we can now plan our hunt for element 120."
It took the team 22 days of operations at Berkeley Lab's 88-inch cyclotron, which accelerates the heavy ions of titanium into a beam powerful enough to fuse with its target. After all that, it yielded just two measly atoms of livermorium.
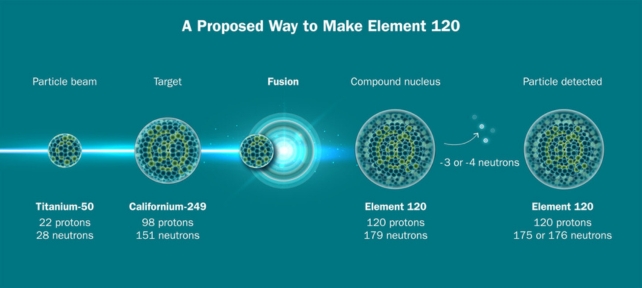
Creating unbinilium with this method, by aiming the beam at californium-249, will be much faster than previous routes could offer, but it's still going to be a slog.
"We think it will take about 10 times longer to make 120 than 116," says Berkeley Lab nuclear physicist Reiner Kruecken.
This marks a return to the superheavy element race for the US Department of Energy's Berkeley Lab, a leader in elemental discovery during the 20th century.
Scientists around the world have been on a race to produce unbinilium since at least 2006, when a Russian team at the Joint Institute for Nuclear Research made a first attempt. Scientists at the GSI Helmholtz Centre for Heavy Ion Research in Germany made several attempts between 2007 and 2012, but no dice.
Now, with researchers from the US, China, and Russia throwing their hats in the ring, one has to wonder what exactly the future applications might be.
"It's really important that the US is back in this race, because superheavy elements are very important scientifically," nuclear physicist Witold Nazarewicz, who was not involved in the research, told Robert Service at Science.
Element 120 is near the theoretical 'island of stability', a paradise for superheavy elements where half lives are luxuriously long, thanks to their 'magic numbers' of protons and neutrons.
These long-lived, stable superheavy elements are expected to offer scientists the chance to study the extremes of atomic behavior, test nuclear physics models, and chart the limits of atomic nuclei.
This paper has been submitted to Physical Review Letters, and is available as a preprint at arXiv.