A cell is not an island. Each one has a host of ways to detect its surroundings, and even physically reach out to neighbors or enemies using strange cellular appendages.
These tentacle-like protrusions are called filopodia, and a new study has given us more insight into how they allow our cells to move about, by twisting the skeleton-like inner scaffolding.
"These structures play a pivotal role in .. allowing cells to explore their environment, generate mechanical forces, perform chemical signaling, or convey signals via intercellular tunneling nano-bridges," the researchers write in their paper.
"The dynamics of filopodia appear quite complex as they exhibit a rich behavior of buckling, pulling, length and shape changes. Here, we show that filopodia additionally explore their 3D extracellular space by combining growth and shrinking with axial twisting and buckling of their actin rich core."
Said core is composed of proteins called actin and myosin. The team, led by biophysicists from the Niels Bohr Institute in Denmark, compares this newly discovered twisting and buckling motion to a rubber band.
When twisted, a rubber band is contracted and suddenly can move on its own, springing back to its original, untwisted configuration. Within the cores of filopodia, myosin proteins coil around actin proteins, pulling them into twists or buckles. The resulting movement allows cell 'tentacles' to sense their environment, interact with other cells or microorganisms, and even move about.
"They're able to bend – twist, if you will – in a way that allows them to explore the entire space around the cell, and they can even penetrate tissues in their environment," says lead author, Niels Bohr Institute biophysicist Natascha Leijnse.
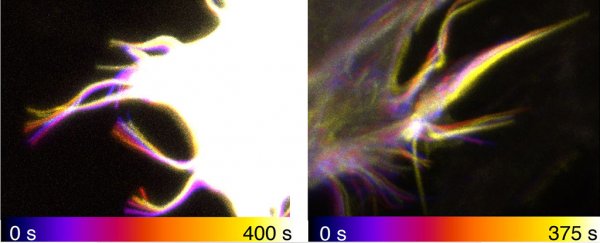
The team used optical tweezers and a confocal microscope to physically watch this actin and myosin shaft twist; afterwards, they built a physical model to confirm that the movement arose spontaneously from these molecules being confined in narrow channels within the filopodia.
Optical tweezers are fascinating bits of tech, where perfectly calibrated laser beams hold a tiny object in place. In this case, an optical tweezer was used on a tiny bead, which the filopodium would grow towards and then get stuck on, holding the 'tentacle' in place.
 (Niels Bohr Institute/University of Copenhagen)
(Niels Bohr Institute/University of Copenhagen)
The researchers used a variety of cells to confirm this wasn't a one-off phenomenon – looking at everything from human breast cancer cells to human embryonic kidney cells.
The presence of these structures in a large variety of cells means it could be another avenue to look into when researching diseases such as cancer.
"Cancer cells are noted for their being highly invasive. And, it is reasonable to believe that they are especially dependent on the efficacy of their filopodia, in terms of examining their surroundings and facilitating their spread," says Niels Bohr Institute biophysicist Poul Martin Bendix.
"So, it's conceivable that by finding ways of inhibiting the filopodia of cancer cells, cancer growth can be stalled."
This will need much more research – currently scientists are just observing this process, but finding out something new about our own cells is always an exciting part of fundamental research.
The research has been published in Nature Communications.