In Paris in the 1930s, letters and small parcels could be delivered via an elaborate network of underground pneumatic tubes that interlaced the city.
Cells inside living fish embryos have been spotted doing a similar thing on a micro-scale. In a study made available on the pre- peer review archive bioRxiv, researchers in France witnessed long, thin tubes shuttling cargo between cells inside zebrafish embryos.
"This study marks the first demonstration of functional tunneling nanotubes in a living embryo," the authors report.
Cells were first seen extending tendrils to other cells in 2004. Since then, cancer cells have been caught using these 'nanotubular highways' like straws to suck up the energy powerhouses known as mitochondria from healthy cells.
In petri dish experiments, tunneling nanotubes that stretch up to 100 micrometers in length seem to provide an important intercellular transport service for chemicals, messenger RNA, proteins, organelles, viruses, and bacteria. These nanotubes could play a role in the development of cancer, Alzheimer's disease, HIV and SARS-CoV2.
Observing mini-postal systems at work in a petri dish is one thing, but it's another thing entirely to verify the same network exists inside a complex, 3D structure like a living animal. Inside multicellular living things, there's so much stuff packed in together that the slender fibers can easily get lost in the noise.
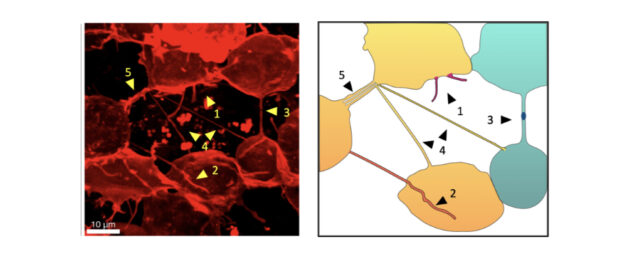
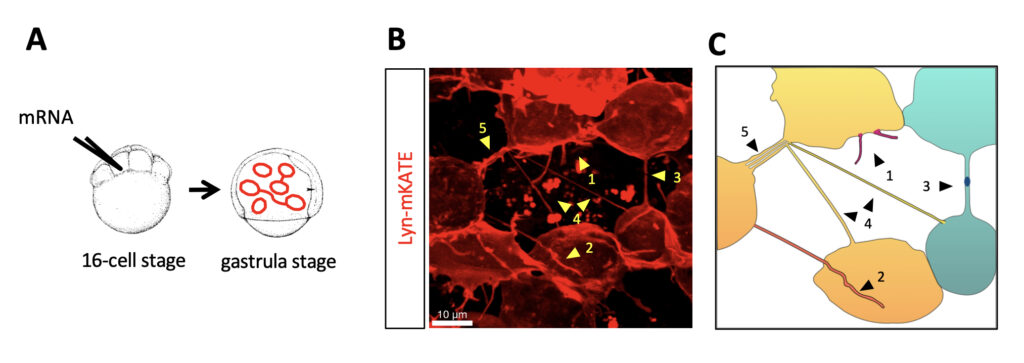
"Cells are very densely packed, which makes it impossible to observe intercellular structures if all of the cells are labeled," the researchers write.
The French researchers overcame this difficulty by tracking the growth of tendrils inside rapidly developing, transparent zebrafish embryos.
At the 16-cell stage of each embryo, they injected one cell with membrane-labeling mRNA. This dye spread in a mosaic fashion as the cell divided – affecting some cells but not others. This lit up a web of thin fibers connecting the cells.
Tunneling nanotubes were distinguished from other tendrils as they formed uninterrupted threads that were longer than 5 micrometers.
Once the embryo reached its gastrula stage, around 35 percent of the labeled cells were connected by tunneling nanotubes.
The movement of soluable materials and bulkier items is a "defining feature of open-ended tunneling nanotubes", the researchers write.
To confirm this trait was present in the fish embryos, the researchers injected the cells with a Dendra2 protein that is too big to pass through other intercellular channels (such as jap junctions between neighboring cells). They then observed signs of the cumbersome protein passing from one cell to another.
The team also injected cells with a mRNA dye that would label mitochondria from one cell and then watched these mitochondria being transported through a nanotube to a far away cell.
This paper was made available as a preprint in bioRxiv.