Cicada wings can kill and remove bacteria, and now researchers have used simulations to study the functions of blunt spikes on their surface, with some surprising findings.
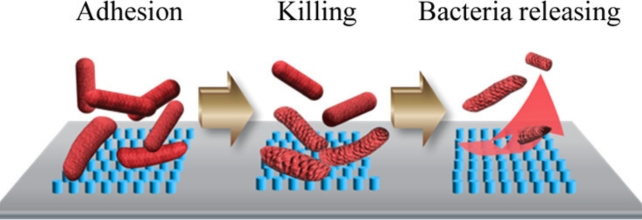
Understanding this natural process could solve a significant healthcare challenge. Medical devices like catheters enable microbial colonization and biofilm formation by providing a surface for bacteria to cling to, so scientists are developing more effective bactericidal surfaces.
Researchers have previously studied the chemical and physical characteristics of cicada and dragonfly wings, but a lot is unclear about their antibacterial properties, like how they remove traces of their bacterial victims.
"At this moment, we know that the cicada wing can prevent bacteria adhesion, but the mechanism is not clear," says Tadanori Koga, a chemical engineer at Stony Brook University in New York.
After reading 2012 research on cicada wings' lethal puncture of bacterial cells, Koga and Stony Brook University polymer physicist Maya Endoh decided to replicate and study the wings' nanopillars.
"The cicada wing has a really nice pillar structure, so that's what we decided to use. But we also wanted to optimize the structure," Koga says.
To mimic the wing of one of these fascinating critters, materials scientist Daniel Salatto from Stony Brook University used a polymer often used in packaging to create tiny structures shaped like pillars on a silicon base.
"The diblock polymer technically can create the nanostructure by itself as long as we control the environment," Endoh says. "Even though we use a common polymer, we can have the same or similar property that the cicada wing column's bactericidal property shows."
Cicada wings have nanopillars that are about 150 nanometers (nm) tall and the same distance apart, but the team tested varying dimensions to see how this would affect the process.
"We thought that the height would be important for the nanostructure because we originally expected that the pillars' height was acting as a needle to puncture the bacteria's membrane," Endoh explains.
During lab testing, they found that surfaces of super-small nanopillars, about 10 nm tall, 50 nm wide, and 70 nm apart, were very effective at killing Escherichia coli bacteria and also releasing them for at least 36 hours, leaving no accumulated dead bacteria or debris on the surfaces.
"It's known that sometimes when bacteria cells die and they absorb onto surfaces, their debris will stay on the surface and therefore make it a better environment for their brethren to come in and absorb on top of them," Salatto explains.
"That's where you see a lot of biomedical materials fail because there's nothing that addresses debris that works well without using chemicals that more or less could be toxic to the surrounding environments."
But they still didn't know exactly how the nanopillars accomplish the double task of killing and removing surface bacteria from the wings.
To understand how these surfaces work, they enlisted the help of Jan-Michael Carrillo, a computational chemist at Oak Ridge National Laboratory in Tennessee, who ran high-resolution molecular dynamics (MD) simulations using a simplified model of the E. Coli bacteria.
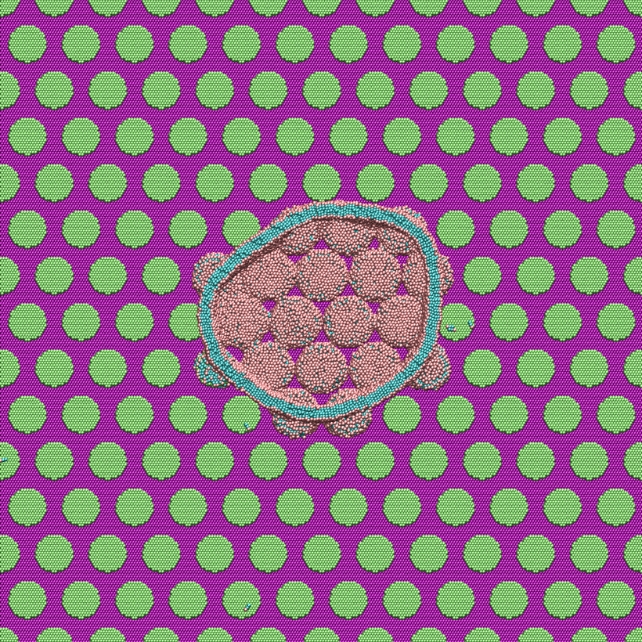
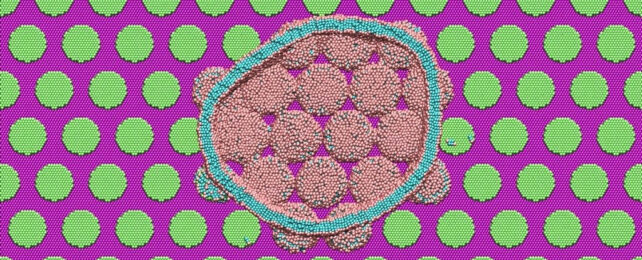
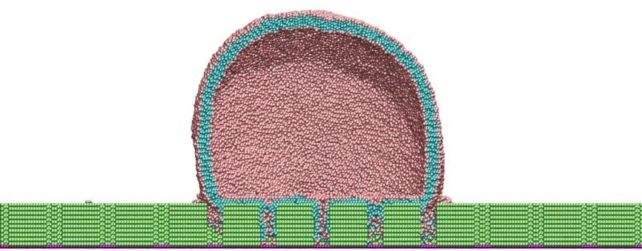
Large-scale MD simulations consisting of about a million particles showed that when the bacteria come into contact with the pillar surface, their lipid outer shell (membrane) has a strong interaction with the nanopillars.
"The lipid heads strongly absorb onto the hydrophilic pillar surfaces and conform the shape of the membrane to the structure or curvature of the pillars," Carrillo explains.
"A stronger attractive interaction further encourages additional membrane attachment to the pillar surfaces. The simulations suggest that membrane rupture occurs when the pillars generate sufficient tension within the lipid bilayer clamped at the edges of pillars."
The membrane continues to be stressed after rupturing, and tension builds until the bacteria detach from the pillars, effectively cleaning the surface.
Adding a thin layer of titanium oxide (TiO2) to the pillars made the bacteria-killing and releasing properties even better, and they also worked against Gram-positive bacteria called Listeria monocytogenes.
Gram-positive bacteria have a less 'stretchy' outer shell, and the stress concentrates more at their attachment points to the pillars, causing them to rupture easily, but their cells didn't appear to have a strong enough attraction to the pillars without TiO2.
Some of the mechanisms need more clarification, but it was surprising to the scientists that the most efficient method wasn't copying nature's design.
"It's not the way we thought," Endoh says. "Even though the nanopillars' height is short, the bacteria still automatically died. Also, unexpectedly, we didn't see any absorption on the surface, so it's self-cleaning.
"This was thought to be due to the insect moving its wings to shake off the debris. But with our methodology and structures, we prove that they just naturally kill and clean by themselves."
The team plans to use further simulations to uncover other mechanisms, especially the self-cleaning function, to eventually improve antibacterial coatings for use in the medical field.
The research has been published in ACS Applied Materials & Interfaces.