Heat production in skeletal muscle triggers the metabolic benefits that come with being exposed to cold temperatures, according to new research, challenging models that see fat tissue as responsible.
In a peer-reviewed comment article, a team of Canadian researchers explain how muscles are the primary heat generator, and drive metabolism of glucose and lipids when temperatures drop.
There's been a fair amount of interest in cold exposure, particularly as a drug-free option for managing obesity and its metabolic complications, as the authors mention. Exercise in cold weather burns more fat during the exercise, and cold exposure uses both skeletal muscle and brown adipose tissue (the 'good' type of fat) to increase energy expenditure.
However, the team writes, "We highlight data that show that skeletal muscle is the primary thermogenic tissue in cold-exposed humans."
Brown adipose tissue (BAT) in humans has been linked to lower body mass indices and a lower prevalence of type 2 diabetes, cardiovascular disease, and nonalcoholic fatty liver disease.
Yet according to McMaster University endocrinologist Logan Townsend and colleagues "few (around 5 percent) adult humans have spontaneously detectable BAT under typical indoor ambient conditions."
Our preference to keep cozy reduces our thermogenesis requirement, but it sure makes interpreting the influence of BAT on metabolic health difficult.
Under chilly conditions, nearly all adults have detectable BAT to varying degrees, and BAT consumes three times more oxygen per gram than cold-stimulated skeletal muscle.
"Given that cold exposure is required to adequately stimulate human BAT," the team writes, "the inference is that stimulating BAT through regular cold exposure protects or reverses metabolic complications."
However, humans don't have much BAT even in the cold, and studies show its thermogenesis contributes to less than 1 percent of energy expenditure in cold-exposed adults.
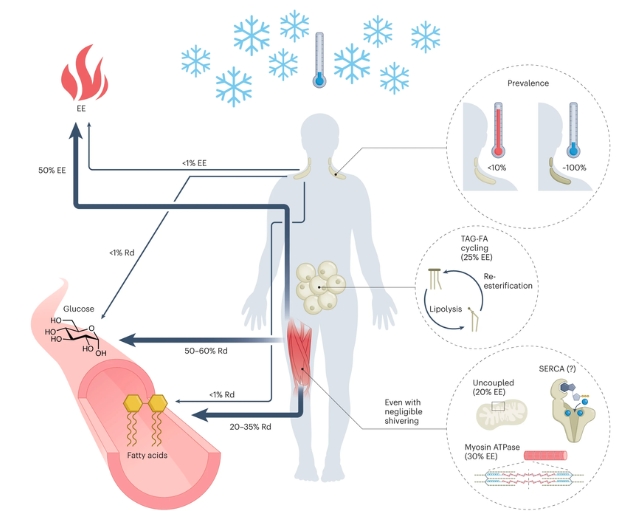
Meanwhile, when we're cold, the shivering mechanism in our skeletal muscles uses energy bound up in molecules of ATP and myosin ATPase activity to produce heat.
Our skeletal muscles use other methods too, accounting for about 50 percent of the energy we use during mild cold exposure. They have even evolved a way to generate heat while at rest.
Thompson and colleagues consider that the remaining energy expenditure in response to cold probably involves multiple other body systems, such as the liver's metabolic activity. And our 'insulating' white adipose tissue (WAT) uses energy in a process to break down and rebuild fats called triacylglycerol-fatty acid (TAG-FA) cycling.
Cold increases glucose use in skeletal muscle and lowers blood sugar in lean people and those with type 2 diabetes. In individuals with obesity and type 2 diabetes, insulin sensitivity increased by about 43 percent after 10 days of periodic cold; the scientists say these effects are mostly attributed to skeletal muscle glucose use.
Studies on mice exposed to cold suggest BAT plays a major role in regulating lipid and glucose clearance, but the scientists point out they have vastly different levels of BAT from humans and BAT is present in rodents regardless of their climate.
The team writes, "BAT is unlikely to directly influence systemic metabolism in cold-exposed humans."
They do stress that BAT has promise as a biomarker of adipose tissue health and may open the door to preemptive disease diagnosis and treatment.
The proposal doesn't rule out cold as the main stimulus for human BAT thermogenesis, nor does it mean cold exposure is definitely beneficial to overall health. More research is needed in this area, with a new approach suggested.
"A concerted effort is needed to move towards a more integrated perspective," the authors conclude, "that simultaneously examines thermogenic adipose tissue, WAT and liver and that particularly emphasizes the organ that is most heavily involved in producing heat and consuming circulating substrates in the cold – skeletal muscles."
The article is published in Nature Metabolism.
