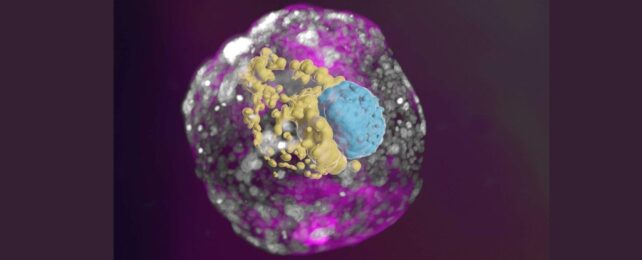
Scientists have developed human embryo models from stem cells cultured in a lab, that gives an unprecedented view of the critical first week after implantation into the uterine wall.
Fertility, early pregnancy loss, and developmental birth defects can all be better understood with a deeper dive into what happens immediately after a fertilized egg burrows into the womb's lining at the start of gestation. But ethical and technical challenges have hampered our ability to study these critical stages of human embryo development.
"The drama is in the first month, the remaining eight months of pregnancy are mainly lots of growth," says molecular geneticist Jacob Hanna from the Weizmann Institute of Science in Israel.
"But that first month is still largely a black box. Our stem cell–derived human embryo model offers an ethical and accessible way of peering into this box."
The international research team coaxed genetically unmodified, undifferentiated human-derived stem cells into complex structures that mimic human embryonic development.
The process reveals the remarkable self-organizing ability of human stem cells, expanding on a recent breakthrough in the generation of embryonic-like stem cells to provide researchers with a new way to study phenomena that have until now been obscured by practical and ethical challenges.
Key features not seen in previous models include the three lineages that make the placenta and embryonic support structures, as well as the layer of cells that form an embryo before it folds in on itself and grows into different tissues and organs.
Previous research showed that stem cells removed from mouse embryos can still be artificially guided to grow into tissues that support and make up the embryo itself, self-assembling into a structural stem-cell based embryo model (SEM) at the post-gastrulation stage where the embryonic cells form into the three main types of body tissue.
"Here, we extend these findings to humans, while using only genetically unmodified human naïve embryonic stem cells," writes Hanna and colleagues.
"We proceeded to test the capacity to form embryo-like structures… that could mimic different stages of natural human in utero development."
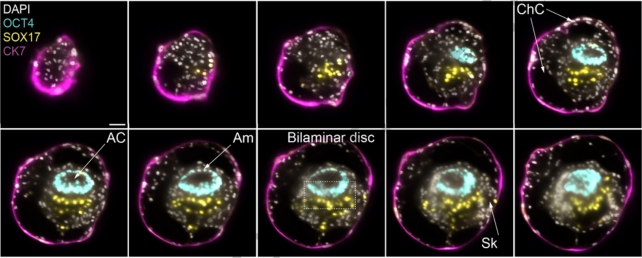
They determined optimal conditions including cell numbers, ratios within cell mixtures, and culture compositions for various stages, starting from when implantation occurs 7–8 days after fertilization.
"These human complete SEMs demonstrated developmental growth dynamics that resemble key hallmarks of post-implantation stage embryogenesis up to 13-14 days post-fertilization," the authors write.
The models depict the assembly of all the known lineages and components of early-stage human embryos, including the epiblast, hypoblast, extraembryonic mesoderm, trophoblast, and the yolk sac.
Cell profiles from the human SEMs dataset were found to resemble the gene expression patterns and cell type composition in human embryos shortly after implantation, when compared to a reference dataset.
Human SEMs are not identical to embryos, the authors note, but they are a model that opens up huge research possibilities.
"Many failures of pregnancy occur in the first few weeks, often before the woman even knows she's pregnant. That's also when many birth defects originate, even though they tend to be discovered much later," Hanna says.
"Our models can be used to reveal the biochemical and mechanical signals that ensure proper development at this early stage, and the ways in which that development can go wrong."
The study has been published in Nature.