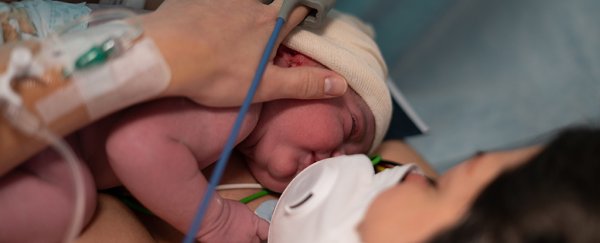
A pregnant woman with suspected COVID-19 was rushed by ambulance to Skåne University Hospital, in Malmo, Sweden, suffering from sudden severe abdominal pain. The doctors noticed that the unborn infant had an abnormally low heart rate, which can be a sign that the baby is not getting enough oxygen.
The doctors performed an emergency caesarean section and delivered the baby within minutes. Blood tests from the baby confirmed it had severely low oxygen, and throat swabs showed that both mother and baby were suffering from COVID.
Using throat swabs from the mother and the newborn, the genome of the virus was sequenced to confirm the possibility that the infant had been infected with COVID while still in the womb.
My colleagues and I – part of a study team at the hospital – found that the viral genome in the mother and the baby was identical. Since the baby had been isolated from the mother directly after the caesarean and had not come in contact with other family members when these tests were done, the findings confirmed that the baby was indeed infected before it was born.
However, a few days later, new genetic sequencing showed that the baby's virus population had changed and contained a mutated version of the virus along with the original virus strain from the mother.
To the best of our knowledge, this is the first case of a genetic change of the coronavirus in the unique setting of mother-to-foetus transmission before birth.
Although it is common for viruses to mutate, this mutation (called A107G) happened just five days after the baby was delivered.
The genetic changes may have been stimulated by the baby coming in contact with the external environment outside the mother's womb. However, it was surprising how quickly this single mutation occurred.
The most important findings were the changes we saw in the placenta. The placenta takes blood and nutrients to the foetus and takes away waste and is critical for the growth and wellbeing of the foetus. We found that half the tissue was damaged.
There was widespread inflammation, and we found coronavirus protein on both the mother's and foetus's side of the placenta. We also found coronavirus protein in all areas that were damaged by inflammation.
The mother made a quick recovery from her COVID infection and was discharged four days after delivery, but the baby needed neonatal care since it was born prematurely (week 34 of pregnancy).
The baby developed antibodies against the virus and had no severe symptoms after delivery. It was, therefore, the baby's own immune system that neutralised the virus as we did not find any antibodies in the mother's breast milk.
Rare but needs monitoring
Our study, which has just been published in The British Journal of Obstetrics and Gynaecology, is among only a handful of scientific papers that have investigated coronavirus transmission through the placenta.
Previous studies have reported rapid placental failure and abnormal foetal heart rhythm, similar to what we found. But with thousands of pregnant women infected worldwide, mother-to-baby transmission in the womb seems to be a rare complication of COVID during pregnancy.
Scientists think that this is because of the placental barrier that protects the baby in the womb from most infections. Also, the vital receptor needed for coronavirus entry into cells, called an ACE-2 receptor, only exists in low levels in the placenta.
In rare cases, coronavirus can damage the placenta – leading to a lack of oxygen in the unborn child – even if the mother has a mild case of COVID in late pregnancy.
Our findings suggest that perhaps we should rethink how we monitor pregnant women who have COVID, and they should be considered a more important risk group than we do today.![]()
Mehreen Zaigham, Postdoctoral Research Fellow, Obstetric & Gynecology, Skåne University Hospital, Lund University.
This article is republished from The Conversation under a Creative Commons license. Read the original article.