Right down on a molecular level, water ice can form out of a rich variety of structures. Though they're all fairly similar in general appearance, differences in their properties means distinct arrangements of solidified H2O can react and behave differently too.
One such phase is called cubic ice, and it is the subject of ongoing scientific debate. In fact, some researchers have even questioned whether it actually exists. Ice that has previously been described as largely cubic may in fact be a mixture containing other, more common types of ice.
In a new study, scientists from the Chinese Academy of Sciences (CAS) and other institutions claim to have created fairly pure cubic ice – or Ice Ic, to give it its technical term. The findings could have implications in everything from climate change to materials science.
"[T]here is a long-standing debate about whether water can freeze to form cubic ice – a currently undescribed phase in the phase space of ordinary hexagonal ice," write the researchers in their published paper.
An ice's phase is set by the environmental conditions – such as temperature and pressure – that are in play when it forms. In cubic ice, water molecules and ice crystals are arranged in a cubic pattern, hence the name.
Using a technique known as transmission electron microscopy (TEM), the team looked at ice forming on 2D sheets of carbon called graphene at very low temperatures. TEM uses beams of electrons and their reactions to image objects at molecular scales.
The ice that formed was primarily cubic ice, with a smaller amount of the more regular, hexagonal ice (Ice Ih) alongside it. That two ice phases formed might give scientists a clue as to why cubic ice hasn't shown up properly in past experiments, the team says.
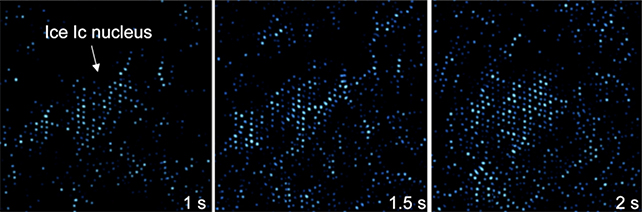
"The observed pure-phase single crystals fully demonstrate the heterogeneous nucleation of pure-phase ice Ic at the current conditions," write the researchers.
No transitions between cubic and hexagonal ice were observed, the team reports, and the cubic ice stayed stable in the conditions of the experiment; 102 Kelvin (-171 degrees Celsius or -276 degrees Fahrenheit). That stability has been an issue in the past.
As this experiment shows, cubic ice is more likely to form at very cold temperatures, far below what you'd manage with your home freezer. That means understanding it is crucial for the study of ice in the upper atmosphere, ice used for cryopreservation, and so on.
The case isn't quite closed yet, and the researchers acknowledge that further investigations are required to confirm the existence and properties of cubic ice – including how its defects might influence its behavior.
"The examination of the structure and dynamics of defects in ice Ic provides a key step towards understanding the plasticity of ice at the molecular level," write the researchers.
The research has been published in Nature.
