A new antibody treatment for endometriosis yet to be tested in humans appears to do what existing therapies cannot: shrink the potentially painful lesions and adhesions that plaster themselves throughout millions of bodies around the world.
Endometriosis is a disease where tissue that usually lines the inside of the uterus grows outside the womb and attaches to other organs in the body. It affects one in ten women of reproductive age, causing debilitating pain, excessive bleeding, and inflammation.
There are other potential therapies in the pipeline which are showing promise in treating endometriosis-related pain with fewer adverse effects than existing drugs.
Hormone therapies are another option to help slow or stop new adhesions from growing, but they cannot make existing lesions go away and also come with unwanted side effects.
While human trials for this new drug candidate are still a way off, results from animal studies suggest it could have a marked effect on endometriosis lesions and associated fibrosis, marked by a thickening and scarring of tissue.
Monthly injections of the antibody therapy shrank endometriosis lesions and reduced fibrosis in monkeys. Several of the test subjects had spontaneously developed endometriosis, but most had endometrial tissue surgically implanted to mimic the human condition.
The study was conducted by a team of researchers led by Ayako Nishimoto-Kakiuchi in the Translational Research Division of Chugai Pharmaceutical Company, a Japanese drug manufacturer.
Nishimoto-Kakiuchi and colleagues developed the antibody therapy, dubbed AMY109, after looking for differences in the expression of nearly 250 genes involved in inflammation in human tissue samples from 10 patients with endometriosis and 4 people without the condition.
In that small set of samples, levels of one particular signaling molecule called interleukin-8 (IL-8), which is a major player in the body's inflammatory response, were closely correlated with the inflammation of endometriosis and disease-linked fibrotic lesions.
That result led the researchers to develop AMY109, a long-acting antibody that binds to and blocks IL-8 signaling. Its sequence is patent-protected, which will limit other researchers' ability to test it in their own experiments.
Antibody therapies typically clear from the body quite quickly, requiring repeated administration. In this case, Nishimoto-Kakiuchi and colleagues report that AMY109 lasts long enough in circulation that a once-monthly injection would suffice.
They tested two different doses of the drug, administered over 6 months, and found that in monkeys with surgically-induced endometriosis, both doses not only reduced the volume of lesions but also diminished fibrosis compared to controls.
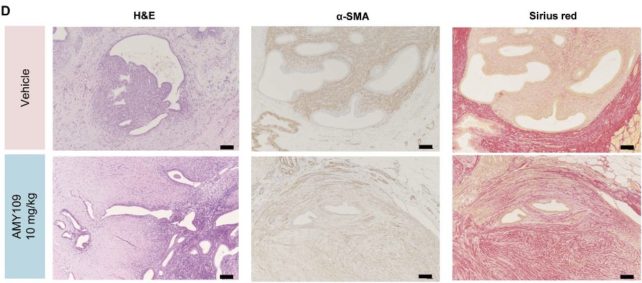
"This was a rather unexpected result, because multiple factors could contribute to fibrosis," the researchers write in their peer-reviewed paper.
That possible link between IL-8 and endometriosis will need further investigating, but as the researchers conclude from these preliminary results, "AMY109 may represent a disease-modifying therapy for patients with endometriosis".
Effective therapies for endometriosis can't come soon enough for thousands of people living with the condition who find surgery only temporarily relieves their pain. In fact, some health experts think repeat surgery to remove out-of-place tissue may be making things worse.
And that's after patients have waited years or even decades to get a diagnosis because their pain was repeatedly dismissed or normalized by the healthcare professionals they sought help from.
More funding for research is sorely needed to better understand the three subtypes of endometriosis that clinicians have known about for decades, and how each subtype responds to treatment.
In 2021, researchers at Oxford University reported the latest findings from their investigations probing the genetics of endometriosis: they identified one gene variant often found in more aggressive forms of the condition, which pushes deeply into surrounding organs and tissues.
More studies like this could lead to better treatments and an understanding of how the disease progresses. The more avenues of research pursued, the better.
The research has been published in Science Translational Medicine.
