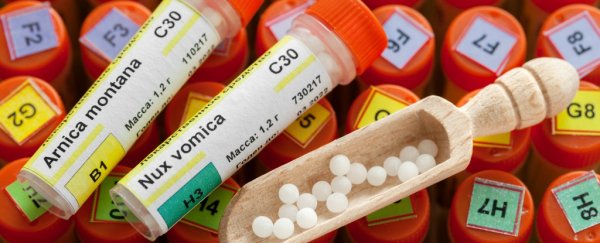
The US Food and Drug Administration (FDA) on Monday proposed a tougher enforcement policy toward homeopathic drugs, saying it would target products posing the greatest safety risks, including those containing potentially harmful ingredients or being marketed for cancer, heart disease and opioid and alcohol addictions.
Homeopathy is based on an 18th-century idea that substances that cause disease symptoms can, in very small doses, cure the same symptoms.
Modern medicine, backed up by numerous studies, has disproved the central tenets of homeopathy and shown that the products are worthless at best and harmful at worst.
Under US law, homeopathic drugs are required to meet the same approval rules as other drugs. But under a policy adopted in 1988, the agency has used "enforcement discretion" to allow the items to be manufactured and distributed without FDA approval.
Agency officials don't plan to begin requiring that homeopathic products get approval - officials say that would be impractical - but they are signalling stepped-up scrutiny for items deemed a possible health threat.
Examples of high-risk products include ones that are administered by injection, are intended for vulnerable populations like children or the elderly, or are marketed for serious diseases, the agency said.
The FDA's proposed approach, outlined in a draft guidance that will be open for public for 90 days, comes more than a year after homeopathic teething tablets and gels containing belladonna were linked to 400 injuries and the deaths of 10 children.
An FDA lab analysis later confirmed that some of the products "contained elevated and inconsistent levels of belladonna", a toxic substance, the agency said.
Once a niche field, homeopathy has grown into to a US$3 billion industry that peddles treatments for everything from cancer to colds, FDA Commissioner Scott Gottlieb noted in a statement.
"In many cases, people may be placing their trust and money in therapies that may bring little or no benefit in combating serious ailments, or worse - that may cause significant and even irreparable harm" because of poor manufacturing quality or unsafe ingredients, he said.
Still, he said, the agency wants to balance its safety concerns with the desires of consumers who want to continue using the products.
Under its planned approach, many products won't be considered high risk and will remain available to consumers, Janet Woodcock, director of the FDA's Center for Drug Evaluation and Research, told reporters during a teleconference.
But she said, the agency would "go after" products that cause - or might cause - "overt harm".
The National Center for Homeopathy, which advocates for homeopathy and is based in Mount Laurel, NJ, says on its website that "homeopathy is a safe, gentle, and natural system of healing that works with your body to relieve symptoms, restore itself, and improve your overall health."
Steven Salzberg, a biomedical engineer at Johns Hopkins University who in the past has criticised the FDA for not taking action against homeopathy, said it was "terrific" that the agency now plans to try to rein in the industry.
He cautioned that product makers are likely to "hit back hard with lots of spurious claims in an effort to confuse consumers and to protect their profits."
Salzberg added that homeopathic products' packaging suggests that the items "cure all sorts of conditions - pain, colds, asthma, indigestion, arthritis, you name it - and yet there's not a whit of evidence" that they cure anything.
The homeopathy field, he said, is "just silly from a scientific point of view, more like a religious belief than a scientific belief."
In July, Britain's National Health System announced plans to stop doctors from prescribing homeopathic drugs. Simon Stevens, the system's chief executive, described homeopathy as "at best a placebo and a misuse of scarce NHS funds".
The move came years after the House of Commons called on the government health service to stop paying for homeopathic prescriptions, saying, "To maintain patient trust, choice and safety, the Government should not endorse the use of placebo treatments, including homeopathy."
In April 2015, the FDA held public hearings on the way it regulates homeopathic products as part of an effort to get public input on its enforcement polices.
The agency said Monday that as a result of the hearing and 9,000 comments submitted by the public, the FDA had decided to propose a new "comprehensive, risk-based enforcement approach to drug products labelled as homeopathic and marketed without FDA approval."
Over the past several years, the FDA has issued warnings about other homeopathic drug products, including zinc-containing intranasal products that may cause a loss of sense of smell; certain homeopathic asthma products that have not been effective in treating asthma and other products that contain strychnine, a poison used to kill rodents.
2017 © The Washington Post
This article was originally published by The Washington Post.