Next time you're having a bad day, feeling sluggish, or simply uninspired about your existence, we have a solution. Watch this video, and remember that within us all are fiery, dancing cells working their butts off just to keep us alive.
Although we've known for decades how cells move throughout organisms, this video is the first high-resolution, three dimensional footage of that process as it's happening. And, holy crap, it's incredible.
This video shows in real time an immune cell migrating through the inner ear of a tiny zebrafish:
Those blue dots you can see the immune cell swallow up are particles of dextran, a sugary polysaccharide that's found in many substances - from wine and heart medication to dental plaque.
In addition to being absolutely mesmerising, this footage is exciting because it's the first of its kind, done using a brand new type of microscopy.
Sure, we've seen cells under the microscope for hundreds of years. But when we try to get footage of them moving, the results are fuzzy.
Our clearest images have always come from groups of cells preserved on glass slides - which meant we were never getting the full story.
"This raises the nagging doubt that we are not seeing cells in their native state, happily ensconced in the organism in which they evolved," says team leader and physicist Eric Betzig, from the Howard Hughes Medical Institute in Virginia.
Even when we try to see just one cell at a time, the cells are blasted with intense light and our microscopes are too slow to follow all the action in 3D.
"This also contributes to our fear that we are not seeing cells in their natural, unstressed form," says Betzig.
"It's often said that seeing is believing, but when it comes to cell biology, I think the more appropriate question is, 'When can we believe what we see?'"
To overcome this, Betzig and his team combined two microscopy technologies: adaptive optics, and lattice light sheet microscopy.
Adaptive optics is the technology astronomers use to see distant celestial objects through Earth's rippling atmosphere.
In the case of imaging a living organism, this means shining a laser on whatever the researchers want to image, and using this to measure how much light distorts as it passes through surrounding cells and tissues.
They then counteract for these interruptions with equal but opposite light distortions, to allow greater visibility in their target area. This is similar to how noise-cancelling headphones work.
The result is a totally clear image of a cell in its natural environment.
The second technique - lattice light sheet microscopy - lets the researchers capture those super-clear images in real time.
It involves rapid and repeated sweeps of ultra-thin sheets of light, which creates a series of 2D images that can be built into a moving high-res 3D picture, all without frying the cell or intefering with its activity.
The result is something scientists have wanted for years - bright, clear, and vibrant images of our cells in action under the microscope.
Even structures inside the cells can be seen using this technique - here are organelles within a zebrafish eye:
 (T. Liu et al./Science 2018)
(T. Liu et al./Science 2018)
This is going to lead to discoveries and breakthroughs we can't even imagine right now.
For example, here's a cancer cell trailing sticky appendages as it rolls through a blood vessel and attempts to hook in somewhere on the vessel wall:
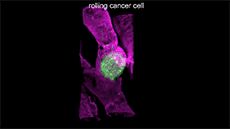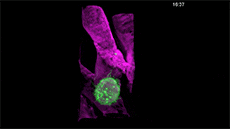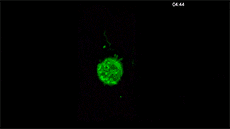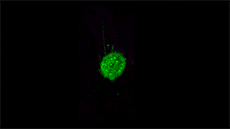 (T. Liu et al./Science 2018)
(T. Liu et al./Science 2018)
The main caveat for all this awesomeness is that right now, this microscopy set up is incredibly expensive and not particularly user friendly. For example, the microscope Betzig and his team uses takes up a 3-metre (10-foot) long table.
But now that we have the proof of concept, the team predicts it's only a matter of time before this technology revolutionises our understanding of biology and human cells.
The team is already working on a smaller and cheaper next-gen version of the microscope. And they'll also share the plans for free, so other labs can make their own.
"It's a bit of a Frankenstein's monster right now," says Betzig. "If you really want to understand the cell in vivo, and image it with the quality possible in vitro, this is the price of admission."
For now, let's just soak up all this incredible footage.
A paper on the new technique has been published in Science.
