Natural diamonds take billions of years to form in the extreme pressures and temperatures deep underground. Synthetic forms can be produced far quicker, but they typically still require some intense squishing for up to several weeks.
A new method based on a mix of liquid metals can pop out an artificial diamond in a matter of minutes, without the need for a giant squeeze.
While high temperatures were still required, in the region of 1,025°C or 1,877°F, a continuous diamond film was formed in 150 minutes, and at 1 atm (or standard atmosphere unit). That's the equivalent of the pressure we feel at sea level, and tens of thousands of times less than the pressure normally required.
The team behind the innovative approach, led by researchers from the Institute for Basic Science in South Korea, is confident that the process can be scaled up to make a significant difference in the production of synthetic diamonds.
Dissolving carbon into liquid metal for the manufacture of diamond isn't entirely new. General Electric developed a process half a century ago using molten iron sulfide, for example.
But these processes still required pressures of 5–6 gigapascals and a diamond 'seed' for the carbon to cling to.
"We discovered a method to grow diamonds at 1 atm pressure and under a moderate temperature by using a liquid metal alloy," write the researchers in their published paper.
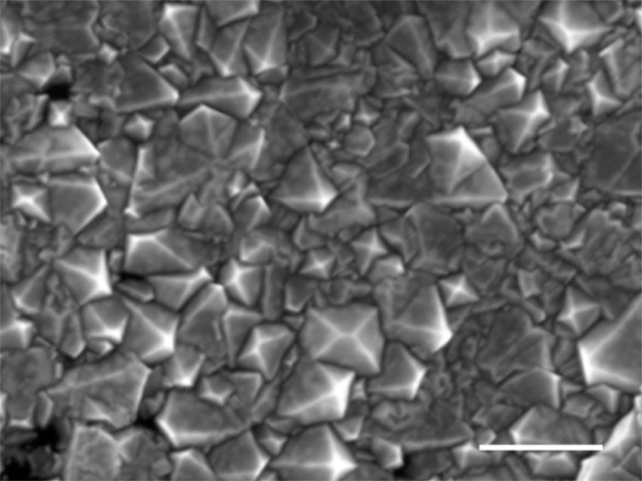
The reduction in pressure was achieved using a carefully mixed blend of liquid metals: gallium, iron, nickel, and silicon. A custom-made vacuum system was built inside a graphite casing to very rapidly heat and then cool the metal while it was exposed to a combination of methane and hydrogen.
These conditions cause carbon atoms from the methane to spread into the melted metal, acting as seeds for the diamonds. After just 15 minutes, small fragments of diamond crystals extruded from the liquid metal just beneath the surface, while two-and-a-half hours of exposure produced a continuous diamond film.
Though the concentration of carbon forming the crystals decreased at a depth of just a few hundred nanometers, the researchers expect the process can be improved with a few tweaks.
"We suggest that straightforward modifications could enable growing diamond over a very large area by using a larger surface or interface, by configuring heating elements to achieve a much larger potential growth region and by distributing carbon to the diamond growth region in some new ways," write the researchers.
Those modifications are going to take time, and research into this process is still at its very early stages, but the authors of the new study think that it has plenty of potential – and that other liquid metals could be incorporated to get similar or even better results.
The process currently used to create most synthetic diamonds – used for a wide variety of industrial processes, electronics, and even quantum computers – takes several days and needs a lot more pressure. If this new technique fulfills its potential, making diamonds is going to become a lot faster and a lot easier.
"The general approach of using liquid metals could accelerate and advance the growth of diamonds on a variety of surfaces, and perhaps facilitate the growth of diamond on small diamond (seed) particles," write the researchers.
The research has been published in Nature.
