Our cells still hold tightly onto many secrets about their origins. But recently discovered giant viruses may hold clues to one such secret - the mysterious emergence of a cell's main store of instructions, the nucleus.
The presence of a nucleus distinguishes our eukaryotic cells from other cellular life forms. Bacteria and archaea tend to simply have a large loop or open stretch of DNA floating free in their cellular juices.
More complex life, like plants and animals, store their genetic material neatly wound around proteins called histones, tucked away in their own cellular compartment, a specialised organelle we call the nucleus.
So how did the bulk of our blueprints get stored in their own little unit?
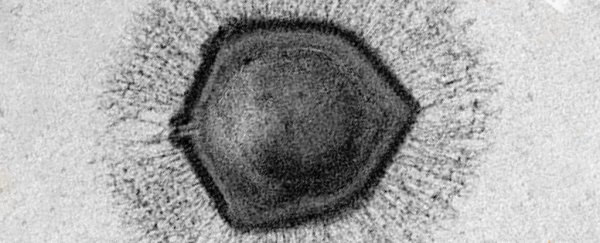
In 2019, Tokyo University virologist Masaharu Takemura and colleagues discovered a giant virus that could force a hijacked amoeba (Acanthamoeba castellanii) cell to form a hard shell-like casing around itself. They named it medusavirus, after the mythical protectress that could turn people to stone.
Now Takemura has proposed a new hypothesis on the evolution of the nucleus involving these bits of protein-packaged DNA, in a review of the current scientific literature.
As with many things in biology, this hypothetical process is both messy and complicated, but Takemura thinks it may have gone down something like this.
Early eukaryotic cells with unbound DNA were infected by giant viruses. These viruses made use of a cell's cytoplasmic membrane, drawing it around tight to protect the viral replication process within the cell.
Over time, the host cell did the same for its DNA to help protect it from viral attack, while also evolving the genes for histones that made their DNA more compact - genes now also found in the viral DNA.
 (Takemura, Frontiers in Microbiology, 2020)
(Takemura, Frontiers in Microbiology, 2020)
Similarities between the membranes that viruses use to replicate within cells, and our nucleus membranes, led researchers to suspect "our ancestral cells devised a viral-like strategy to protect its genome against the assault of viruses."
The medusavirus doesn't make its own bubble to replicate within, like other viruses such as polio do. Instead, it finds its way into the cell's nucleus and uses the host's machinery to replicate. Thus, both the viral and host genome can co-exist during replication.
But the medusavirus does have histone and DNA polymerase genes just like its amoeba hosts, suggesting these genes transferred between the viral genome and the host.
This DNA transfer between virus and eukaryotic cells likely went the other way, too. Researchers believe the amoeba acquired genes for the major capsid protein from the virus.
"I speculate that a portion of viral DNA replication may have aided host genome replication and proved evolutionarily advantageous," Takemura wrote.
The unusually large amounts of genetic material of giant viruses, like poxviruses, code for more than 400 proteins. Some of these include proteins found also in eukaryotes, like the protein that caps messenger RNA but is not found in bacteria or archaea.
Molecular analysis of poxvirus DNA polymerase has also found it is closely related to eukaryotic DNA polymerase.
There are still many parts of this hypothesis that need testing, Takemura points out. But it does align with the leading theory of nucleus evolution - that nuclear membranes came from the inner membrane of early eukaryotic cells - and it helps to explain some of the processes that may have been involved.
"This new updated hypothesis can profoundly impact the study of eukaryotic cell origins and provide a basis for further discussion on the involvement of viruses in the evolution of the eukaryotic nucleus," Takemura said.
This research was published in Frontiers in Microbiology.