When the United States dropped an atomic bomb on Hiroshima in August 1945, the Japanese city was engulfed in a devastating fireball that killed an estimated 140,000 people and vaporized earth and infrastructure.
Seventy years later, scientists discovered fallout debris from the nuclear blast in the form of glassy spheres strewn along the beach of Motoujina, a small island in Hiroshima Bay. The concrete and steel that once made up Hiroshima's buildings had been whipped up and torched in the extreme heat, scientists proposed, before cooling and falling back to Earth as rounded, glass-like beads.
Now, a new analysis of those so-called Hiroshima glasses has revealed how they formed: by condensation within the nuclear fireball.
The glasses' chemical and isotopic composition, analyzed by astrochemist Nathan Asset of Paris Cité University and colleagues, also shows similarities to primitive meteorites called chondrites, which formed from interstellar dust and nebular gas in the early Solar System.
"The formation of the Hiroshima glasses by condensation implies that they may be an analog to the first condensates in the Solar System," the researchers write in their paper.
These first condensates, or solids, also known as calcium-aluminum-rich inclusions (CAIs), contain lots of oxygen-16 isotopes (16O) too, a 'lighter' form of oxygen with fewer neutrons than heavier varieties.
Scientists think these 16O isotopes may have been produced by UV penetrating the interstellar dust-gas cloud from which our primordial Solar System's first chondrites formed, or they could have been produced by specific mechanisms when the vaporized material condensed into liquid before solidifying further.
Only a few lab experiments have tested this second explanation, so studying the fallout debris of the Hiroshima blasts could provide new insights, Asset and colleagues reasoned.
The team analyzed samples collected from the sandy beaches of Hiroshima Bay in 2015 by retired geologist Mario Wannier and his team.
Analyzing 94 pieces of nuclear fallout debris, Asset and colleagues identified four different types of Hiroshima glasses: melilitic, anorthositic, soda-lime, and silica.
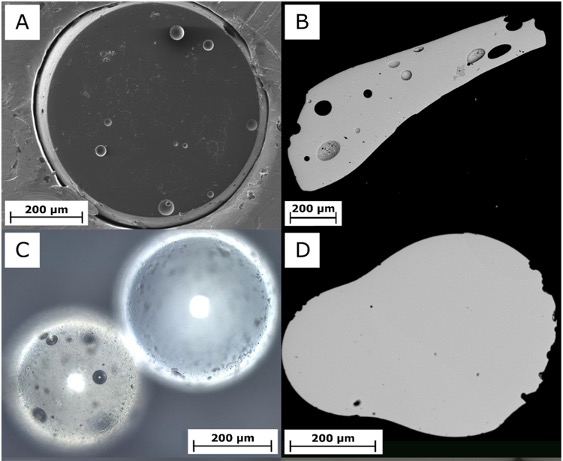
Chemically, the silica glass looked the same as quartz sand grains you'd find on any beach, and the soda-lime glass resembled industrially made glass. However, all four types of Hiroshima glass had "very peculiar" compositions of oxygen and silicon isotopes, giving the researchers a new way to study how they possibly formed.
To take a closer look, the team ran simulations reconstructing the chemical makeup and physical conditions of the nuclear blast from previous research, using those crude estimates to model possible condensation processes within the Hiroshima fireball.
Previous research has estimated that the Hiroshima bomb exploded 580 meters above the city, too far from the surface to leave a crater. Yet temperatures were so intense – reaching 10 million degrees Celsius within the fireball itself and an estimated 6,287 °C (11,349 °F) on the ground – they vaporized building materials in a matter of seconds.
The team's simulations revealed how melilitic liquids condensed from the gas cloud first, in a process known as fractionated condensation, followed by anorthositic, soda-lime, and silica liquids. These droplets then quenched to glasses when they were exposed to temperatures between 1,800 and 1,400 °C depending on their composition.
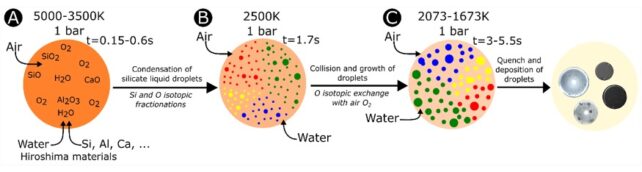
"The melilitic glasses are the first liquid to condensate and the last to quench, so they are the ones which can interact the most with the materials in the fireball," Asset and colleagues explain. "This could explain why a majority of the inclusions are found in this type of glass."
While the researchers are also intrigued by the prospect of peering into the early Solar System through the Hiroshima glasses, they do acknowledge the pressure, temperatures and gaseous mixtures differ massively between the Hiroshima fireball and the solar accretion disk, where chondrites first formed.
"Despite all these differences, the similarities between the Hiroshima glasses and the CAIs could indicate a similar process, namely chemical reactions during condensation, to explain their similar 16O-enrichment," the team concludes.
The study has been published in Earth and Planetary Science Letters.
