For millennia, humans have been trying to stretch our lifespans as far as they will go. We've tried freezing, fasting, and herbal remedies galore.
Recently, billionaires like Larry Page, Mark Zuckerberg, and Jeff Bezos have invested hefty sums into biotech companies like Altos Labs, Juvenescence, and Unity Biotechnology, which are pursuing the quest for longevity through cell rejuvenation and disease prevention.
So far, the longest anyone has ever clung to life is 122 years. But that may be on the lower end of our potential limit.
Humans may have a lifespan limit of 150 years
Even if you lived in a bubble with no disease or danger, your body would still experience wear and tear as it pumped blood, digested food, and conducted all the functions necessary for survival.
The older you grow, the longer it would take for your body to "bounce back" from this wear and tear, because aging is baked into our cells and DNA. All this means your tissues gradually lose the ability to heal themselves, which can lead to disease and dysfunction.
One study suggested the human body's recovery time doubles every 15 years – so a bruise that took one week to heal at age 40 might take two weeks at age 55. Eventually, the human body loses all of its resilience, so whatever bones or tissues break stay broken. Once too many body parts malfunction, you die.
Researchers don't necessarily agree on the maximum limit for when this happens. Some have proposed 115 years, others 130 years. One of the most recent studies analyzing over half a million people in the US and UK suggested humans lose all resilience sometime between ages 120-150.
The big question becomes: What if we could slow that wear and tear, or better yet, prevent it altogether? Some experts argue that with medical advancements, average human lifespans have no natural limit.
Let's take a look at aging on a cellular level, what's holding us back from longer lives, and the groups of researchers looking to understand and potentially reverse the aging process.
Cellular senescence is one of the most researched topics of aging
Cellular senescence is when a cell stops reproducing but doesn't die.
When this happens, some senescent cells turn into destructive zombies, floating around and releasing inflammatory chemicals that harm healthy cells, including stem cells – your body's "repairmen" that help replace damaged or broken tissue.
But not all senescent cells are bad.
Some senescent cells secrete chemicals that help repair wounds, said Paul Robbins, the associate director of the Institute on the Biology of Aging and Metabolism and the Medical Discovery Team on the Biology of Aging at the University of Minnesota.
Companies like Life Biosciences and Unity Biotechnology are currently developing drugs called senolytics to contain and destroy only the "bad" senescent cells in your body. Some experimental drugs may even prevent cells from becoming senescent in the first place.
But so far, no one has figured out how to completely prevent or eliminate harmful senescent cells.
By age 60, the human body – in particular the immune system – has a harder time clearing out harmful senescent cells, which can lead to a build-up that triggers tissue damage and failure, Robbins said.
One major cause of cellular senescence is damage to your DNA, which has helped spark another area of research that led to a Nobel Prize in 2009: telomeres.
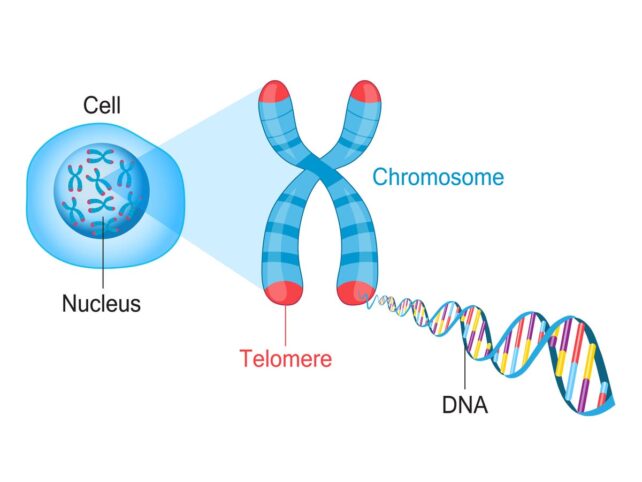
Telomeres help estimate your biological age
Some argue that biological age – how old your cells and tissues are – is a better predictor of your lifespan than your chronological age, or how many years you've been alive.
A common way scientists estimate biological age is to measure the telomeres in certain immune cells.
Telomeres are protective caps at the end of your DNA. They're made up of strings of molecules called base pairs. As you age, those base pairs disappear, shortening your telomeres. And shorter telomeres leave DNA more vulnerable to damage and the effects of aging.
When you are born, the telomeres in certain immune cells, called leukocytes, can have between 7,000 and 11,600 base pairs. Once that size shrinks to 5,000 base pairs, you're at high risk of imminent death, a recent study found.
But other research has found that some people who live to be over 100 actually experience telomeres that get longer each year, not shorter. This has led some scientists to research ways to mimic this telomere recovery process in younger individuals.
For example, Aviv Clinics ran a study examining how 35 older adults responded to hyperbaric oxygen therapy, in which you rest in a chamber with high air pressure and oxygen levels. They managed to increase telomere length in participants' leukocyte cells after 30 daily HBOT sessions.
But most telomeres stopped growing after the 30th session, and scientists don't know yet how long the treatment effects might last.
DNA methylation is linked to several age-related diseases
Another contributor to DNA damage and cellular senescence is DNA methylation – when molecules called methyl groups attach to certain sections of your genes to manage their behavior.
Depending on the location, the methyl groups may block genes from activating or enhance gene activity where necessary.
In general, DNA methylation decreases as you get older, which may allow the wrong genes to activate.
Research has linked methylation decline to several age-related conditions, including Alzheimer's disease, cardiovascular disease, and cancer – though it's worth noting not all methylation changes are bad.
Similar to telomeres, DNA methylation is another way scientists can measure your biological age to help predict your life expectancy. For example, you may have celebrated your 55th birthday, but after years of smoking, your cells may have a level of methylation usually seen in 60-year-olds, thus potentially shortening your lifespan.
Traditionally, DNA methylation tests have used blood, but companies like Elysium Health and research projects like GrimAge have recently developed saliva tests, as well.
People whose methylation age is at least 5 years greater than their chronological age have a 16 percent higher mortality risk, research has found, meaning they are more likely to die from any cause than their same-age peers.
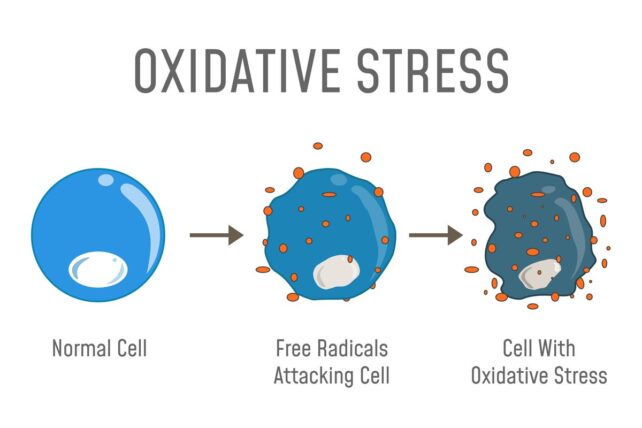
Mitochondria and free radicals are some of the biggest obstacles to longevity
Last but not least, some of the biggest limiters of the human lifespan are the tiny, bean-shaped mitochondria in your cells. These microscopic structures generate most of a cell's energy, which is vital to survival, but also creates byproducts called free radicals.
Free radicals are basically unstable atoms that bounce around and hurt parts of your cell, leading to damage called oxidative stress. Over time, oxidative stress accumulates, causing age-related diseases like Parkinson's, Alzheimer's, and cancer.
Biotech companies like Altos Labs are working on a way to prevent these diseases by rejuvenating cells and undoing the damage that oxidative stress can cause. The company hopes that by resetting cells to a healthier, younger state, it could boost longevity.
The quest for longevity has no single solution
For every mechanism that contributes to aging, there are groups of people working to understand and possibly reverse those processes.
But it's important to note that the aging puzzle has no single solution.
"All of these things that go wrong with aging are linked," Robbins said.
For example, telomere shortening can lead to DNA damage, which in turn disrupts your mitochondria. The free radicals from your mitochondria can in turn damage more of your telomeres and DNA. All these processes mutually influence one another.
No one aging mechanism is more important than the others. That's why all anti-aging research, no matter how niche, is a connected part of humanity's larger goal: to stay alive as long as we can.
This article was originally published by Business Insider.
More from Business Insider: