Little blobs of human brain tissue transplanted into rats have just passed a major milestone in the pathway towards a new way to heal serious brain injuries.
The grafted human mini-brains didn't just integrate with the surrounding rat brain tissue – the neurons in the organoids started to respond to visual stimuli: black-and-white images and lights shone into the rats' eyes.
And this happened within a three-month time period.
"We were not expecting to see this degree of functional integration so early," says physician and neurosurgeon H. Isaac Chen of the University of Pennsylvania.
"There have been other studies looking at transplantation of individual cells that show that even 9 or 10 months after you transplant human neurons into a rodent, they're still not completely mature."
Splicing bits of human brains, in this case known as called cortical organoids, into the brains of rodents is growing increasingly sophisticated. First, it was individual neurons; more recently, scientists have successfully transplanted human cortical organoids into the brains of baby rats and adult mice that joined with the surrounding tissue and showed signs of functionality.
Now, Chen and his team have taken the next step: transplanting human brain tissue into adult rats with large cortical injuries, to see if they, too, can show functional integration.
"We focused on not just transplanting individual cells, but actually transplanting tissue," Chen says.
"Brain organoids have architecture; they have structure that resembles the brain. We were able to look at individual neurons within this structure to gain a deeper understanding of the integration of transplanted organoids."
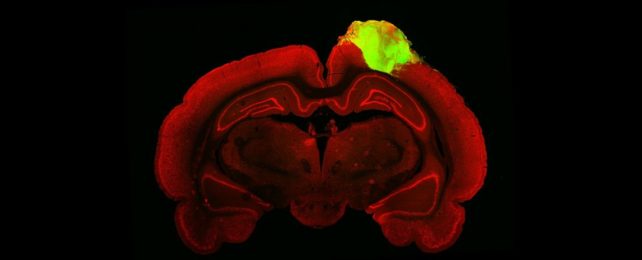
To grow the human mini-brains, the researchers used induced pluripotent stem cells genetically modified to express green fluorescent protein.
Induced pluripotent stem cells are generated from adult stem cells reverse engineered into an embryonic-like undifferentiated state; that is, they can develop into many different types of cells. The green fluorescent protein gives the organoids the ability to fluoresce.
These stem cells were grown into human neurons over the course of about 80 days, developing into small organoids. Once the organoids were grown, the researchers set about transplanting them into the brains of 10 adult male rats.
The researchers first created a cavity in the brain of each rat the size of the organoid, about 2 millimeters across; this cavity represented a serious brain injury. Once the cavity was created, the organoid was inserted, and the rats stitched up, and allowed to heal.
To see how the organoid integrated with the brain after healing, the researchers injected the rats' eyes with fluorescent-tagged viruses that traveled along their synapses. They were then able to trace the neuronal connections from the rats' retina, all the way back to the brain transplanted organoids.
Then, while the rats were shown flashing lights and images consisting of alternating black and white bars, the researchers used electrodes to study activity within the organoid. Around 25 percent of the human neurons responded to the light stimulation.
"We saw that a good number of neurons within the organoid responded to specific orientations of light, which gives us evidence that these organoid neurons were able to not just integrate with the visual system, but they were able to adopt very specific functions of the visual cortex," Chen says.
The experiment was limited to three months because of the limitations of the immunosuppression required to keep the rats' bodies from rejecting the human tissue. At the conclusion of the experiment, the rats were euthanized.
Because of this short time, it's possible that the human neurons were not fully mature. This could explain why the responsiveness of the neurons wasn't higher, the researchers say.
However, the results show promise for this line of enquiry, and can be used to design and refine future experiments. The team recommends using genetically immunosuppressed rodents for longer term studies.
"Neural tissues have the potential to rebuild areas of the injured brain," says Chen.
"We haven't worked everything out, but this is a very solid first step. Now, we want to understand how organoids could be used in other areas of the cortex, not just the visual cortex, and we want to understand the rules that guide how organoid neurons integrate with the brain so that we can better control that process and make it happen faster."
The research has been published in Cell Stem Cell.