Although we constantly use our brains without having to think about it, working out how they do what they do is a much trickier matter. To help figure it out, scientists have for decades resorted to using animal models for brain experiments.
In fact, most knowledge about how the development of the cerebellum - the brain area that receives and coordinates sensory information and our physical responses to it - comes from mice. And a new study suggests that might be an issue.
While humans clearly have a larger volume of brains than mice, new research shows comparisons to such animal models may be fraught with greater challenges than we previously realised, because of some fundamental differences in how our brains develop.
"Our results indicate the presence of many stark differences in developmental patterns between the mouse and human cerebellum," neuroscientists Parthiv Haldipur and Kathleen Millen from Seattle Children's Research Institute told ScienceAlert in an email.
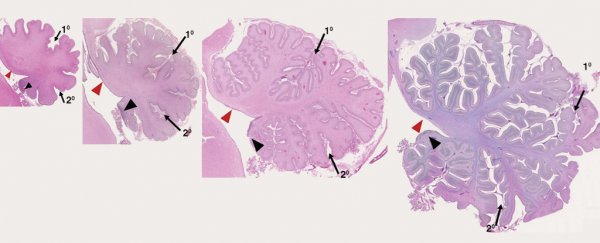
Haldipur and Millen, along with a team of international researchers, looked at how the cerebellum develops in humans between 30 days after conception to 9 months after birth, and compared it with mice and macaques - primates frequently used in brain research.
By examining and comparing these developing brains' morphologies and molecular expressions they found entire groups of brain cells in humans, not seen before in this region of the brain in either mice or macaques.
"These have never been described in any other vertebrate and appear to be human-specific," said Haldipur and Millen.
They are a type of progenitor cells, like stem cells that give rise to other cells, but more restricted in the type of cell they can form.
The researchers describe these cells as outer-radial glial-like basal progenitors (what a mouthful!).
They were previously only known to be present in the mammalian cerebral cortex - the outer neural layer of our brains involved in memory, thought and language. This is the first time they've been spied in the cerebellum.
The study found that in one area of the developing human cerebellum called the rhombic lip, these progenitor cells become the primary source of a brain cell called the cerebellar granule neuron.
These are our most abundant type of neurons, making up 80 percent of all the neurons in the human brain.
"In humans, we find that rhombic lip development is protracted and it is present throughout gestation, including during the 3rd trimester when human cerebellar volume increases 5-fold," said Haldipur and Millen.
Recent studies suggest this expansion of the rhombic lip may be linked to human cognition, Haldipur and Millen explain.
"Perhaps persistence of the human rhombic lip ensures proper growth of this critically important region of the human cerebellum," they said.
These discoveries make already tentative conclusion from animal models used to study human neurology even more shaky. But they also explain some neurological puzzles.
"While many features of cerebellar development are conserved and mice have provided many answers, several human cerebellar birth defects have been difficult to model," Haldipur and Millen explain.
This includes a neurological condition called Dandy-Walker malformation, which occurs when the rhombic lip fails to develop properly. It leads to problems with movement, intellect, moods, and other neurological functions.
"It is now clear that the underlying pathological mechanisms [of Dandy-Walker malformation] can never be fully modelled in mice, because mice lack critically important transient developmental zones seen in humans," Haldipur and Millen reveal.
The mouse samples used in the study were taken from lab animals, the macaque research was done via images from the MacBrain resource, and the researchers used 100 samples of human tissue taken from hospitals and institutions with access to donated tissue.
While the researchers don't know what that the absence of this group of progenitor cells in macaques means for other primates, this study highlights the need to have a closer look at these developmental differences across all species we use to study neurology.
"Our studies underline the urgency of further… analyses of human and mouse cerebellar development to better define the value and limitations of mouse genetic models of human neurodevelopmental disorder," the researchers conclude in their paper.
They are now working on learning more about these newly identified areas of progenitor cells in the hope of developing better diagnostics and treatments for neurodevelopmental disorders.
Their study has been published in Science.