Imagine if lost, degenerated, or diseased parts of the brain could be regrown in the lab and transplanted for a new lease on life. Scientists at the University of California San Diego have gotten us closer to that reality.
Human cortical organoids (or 'mini-brains') transplanted into mice not only connected with the host's vascular system, they reacted to pulses of light shone into the test subjects' eyes in similar ways to the surrounding brain tissue.
Over the course of several months, researchers used an innovative imaging system to measure electrical activity in the organoid that indicated an integrated response to visual stimuli.
It's the first time scientists have been able to confirm functional connections in a transplanted human brain organoid in real time, largely thanks to improvements in implants capable of measuring subtle neurological signalling on a fine scale.
"We envision that, further along the road, this combination of stem cell and neurorecording technologies will be used for modeling disease under physiological conditions at a level of neuronal circuits, examination of candidate treatments on patient-specific genetic background, and evaluation of organoids' potential to restore specific lost, degenerated, or damaged brain regions upon integration," the authors write.
The team of engineers and neuroscientists, led by neuroengineer Duygu Kuzum, developed their new recording system to measure brain wave activity at both a macro and micro level at the same time.
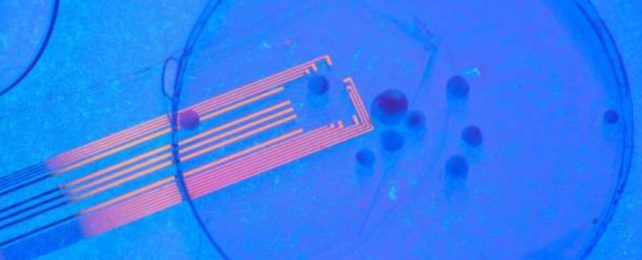
The setup uses flexible and transparent microelectrodes made from graphene that can be implanted into certain parts of the brain. This highly-tuned tech accurately displays spikes in neural activity from both the transplanted organoid and surrounding brain tissue as they occur.
Less than a month after transplantation, researchers found their human organoids had formed functional synaptic connections with the rest of the mouse visual cortex.
Two months later, the foreign tissue had integrated with the host's brains even further.
Previous studies, some conducted by the same authors at UCSD, have shown that human mini-brains implanted into mice can connect to blood vessels supplying oxygen and nutrients. The neurons also start to mature and self-organize.
In 2019, for instance, scientists grew pluripotent stem cells into a pea-sized blob of two million organized neurons that probed its surroundings for neighborly connections.
Pluripotent stem cells also form the foundation of human brain organoids. They have the potential to differentiate into a wide variety of tissues and organs, but only if they are bathed in the right cocktail of molecules. But that mixture is incredibly complex and based on very specific timing, which scientists are still working out.
In 2021, headlines were made when a brain organoid started to develop rudimentary eye structures, and yet the feasibility of achieving functional 'sight' in a lab-grown brain is still a long way off.
Implanting a human brain tissue grown from stem cells into a developed visual cortex, on the other hand, could be a more realistic goal. Studies have achieved this before in rodents, but whether the foreign graft is actively receiving functional input from the rest of the brain has been harder to determine.
Conventional metal electrodes do not give a clear field of view to the brain, which means scientists have to remove the electrodes to properly see the sensory cortex, and this can mess with the success of a tissue graft.
Transparent electrodes help solve that problem. Using a fluorescent imaging technique under the microscope, researchers at UCSD have shown that pulses of light can stimulate transplanted human organoids within a mouse brain.
"We envision that, further along the road, this combination of stem cells and neurorecording technologies will be used for modeling disease under physiological conditions; examining candidate treatments on patient-specific organoids; and evaluating organoids' potential to restore specific lost, degenerated or damaged brain regions," says Kuzum.
The study was published in Nature Communications.