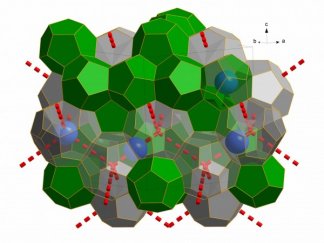
Water molecules can sometimes form crystal structures called a clathrate hydrate by surrounding impurities, such as methane or carbon dioxide, with frozen 'cages'.
Scientists realised that if they could extract the 'guest' molecules they could make a new type of ice. However, this was difficult to achieve because the ice structure is fragile without these extra molecules.
Now, a team at the University of Göttingen in Germany have done just this using a vacuum pump at low temperatures. The trick was to mix in neon atoms, which are relatively small and easily coaxed out of the solid ice. Their research was published in Nature this week.
"What is the point of a novel form of ice?" we hear you say. Scientists believe the study will help remove pipe-blocking clathrates from natural gas and thereby improve the energy efficiency of extraction.
The team of scientists say that if they can figure out how to replace energy-rich methane trapped in the huge amounts of clathrates on the ocean floor with carbon dioxide, then they could harvest energy from the ocean.
According to the authors, "It is important to note that clathrates could also be formed with carbon dioxide gas which would create stable compounds on the ocean floor. This means there is a possibility we could extract methane and convert it to useful energy, and replace it with the CO2."
"In other words, we could pump CO2 down to the ocean floor as a replacement for the methane in the gas hydrates. The challenges involved would naturally be large and the feasibility has been called into question, but it remains an extremely intriguing possibility worth exploring."