To perform a myriad of functions inside our body's cells, many types of hard-working protein slip into and out of dense droplets called condensates to accelerate biochemical reactions as needed.
But this fluidity can crumble in diseases – such as Alzheimer's disease, Parkinson's, and amyotrophic lateral sclerosis (ALS) – which are marked by solid aggregates of lumpy proteins that form in nerve cells.
Now, a team of researchers has developed a new approach for imaging the moment when proteins known to aggregate in neurodegenerative diseases begin to mass together.
Forming hallmark structures like clumps, plaques, and tangled fibrils, "the proteins no longer exhibit rapid reversibility back to liquid form," explains protein biophysicist Yi Shen, of the University of Sydney, who led the study.
"It is therefore crucial to monitor condensate dynamics, as they directly affect pathological states."
Past research has shown proteins that aggregate in ALS, a debilitating disease affecting motor function, exist in a 'supersaturated' state at very high concentrations that exceed their typical solubilities. In other words, these proteins are teetering on the edge as soluble forms, and are prone to solidifying if the cell becomes overwhelmed.
To take a closer look at the behaviors of proteins like these, Shen and colleagues developed two new ways of closely monitoring the transition of a protein from its liquid to solid phase.
Their first test was for a DNA/RNA binding molecule called fused in sarcoma (FUS) protein, which aggregates in ALS and frontotemporal dementia.
When proteins like FUS concentrate as a gel in condensates, a dense, protein-rich phase is surrounded by a dilute one depleted of molecules. Bringing so many proteins close together can tip the mixture towards aggregating even further, irreversibly into solid clumps.
The researchers imaged solutions of FUS condensates as they formed over 24 hours, using two approaches which collected the light refracted through, and scattered back from, the dense protein 'balls'. The patterns reflected the internal structures and density of the condensates, which solidified after five days.
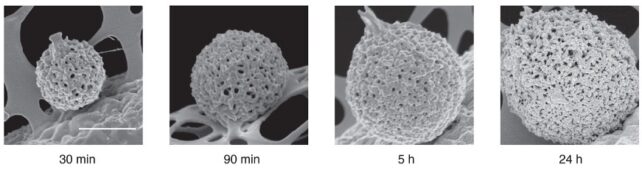
"We use a fast camera to record long bright field sequences of images at a high frame rate. This allows us to explore simultaneously fast (acquiring at high frame rates) and slow dynamics (by acquiring for a long time)," Shen and colleagues write in their published paper.
Like an explosion in the night, you can see the fluorescent green proteins emerge out of the blackness as they aggregate together, appearing to pull more and more proteins out of solution at the condensates' edges until the whole mass seemingly implodes.
If you missed that in the time-lapse video above, watch closely again: it shows the transition from liquid to solid protein starts at the outer edge of the spherical condensate, which thickens and migrates inwards to the core until the whole droplet becomes a solid-like gel.
"This is a huge step forward to understanding how neurogenerative diseases develop from a fundamental perspective," says Shen.
"We can now directly observe the transition of these critical proteins from liquid to solid at the nanoscale – a millionth of a metre in scale," adds Daniele Vigolo, a biomedical engineer also at the University of Sydney.
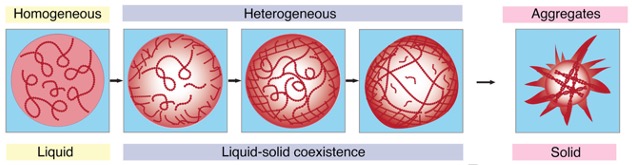
While the experiments were conducted in solutions of lab-made proteins, and there's obviously a whole lot more going on inside cells, the researchers say their findings provide new insights into the fundamental, physical process underlying neurodegenerative diseases.
If the imaging techniques can be replicated with other proteins, we might just learn a whole lot more about the strange ways these proteins interact.
The study has been published in PNAS.
